Neurologia
Túnel carpiano agudo y parálisis nerviosa: patologías de la mano no descritas en pacientes covid
Una serie de pacientes sugiere que el SARS-CoV-2 causaría compresiones nerviosas agudas en los nervios mediano y cubital asociadas a problemas en la mano

Anosmia, ageusia, parálisis facial y óculo-motora… el ‘rastro’ que deja el nuevo coronavirus en el sistema nervioso periférico, una vez resuelta la infección, parece no tener fin. Cada día se descubre una nueva patología asociada a esta infección viral. Para el traumatólogo Juan González del Pino, experto en cirugía de la mano, muñeca y microcirugía dela Policlínica Nuestra Señora del Rosario, en Ibiza, el virus también puede afectar los nervios de la mano, produciendo desde una parálisis de instauración rápida a un síndrome del túnel carpiano agudo.
Así lo ha constatado en al menos ocho pacientes atendidos recientemente. Todos habían superado una infección por SARS-CoV-2, la mayoría con síntomas leves, como pérdida de olfato o gusto, síntomas catarrales, fiebre o dolores musculares. Sin embargo, todos terminaron al cabo de unos meses de la enfermedad con neuropatías localizadas en el codo y la mano.
El cirujano describe cuándo empezó a darse cuenta de la posibilidad de esta nueva patología: “En octubre de 2020, atendí a un hombre de 72 años con atrofia de la musculatura de ambas manos que se había producido en un brevísimo periodo de tiempo. Tenía una parálisis completa bilateral del nervio cubital, responsable de la sensibilidad de los dedos anular y meñique, del movimiento fino y preciso de los dedos, y de parte de las funciones de la pinza del pulgar. Además tenía un síndrome del túnel carpiano en ambas manos de inicio después de su enfermedad. Me aseguraba que hacía 3-4 meses no tenía esas deformidades, ni atrofias y que utilizaba las manos con total normalidad. Al preguntarle sobre enfermedades previas o actuales me indicó que había sufrido infección por coronavirus. Empecé a relacionarlo con la situación clínica y descubrí que había una causa-efecto entre el covid-19 y las neuropatías que el paciente presentaba”.
La historia se repitió en noviembre con varias mujeres de entre 45 y 65 años, que acudieron a su consulta con una evolución idéntica y que igualmente habían estado infectadas por el coronavirus. “El patrón se repetía: enfermedad en la primera ola y desarrollo de atrofias musculares en verano, o enfermedad en la segunda ola y establecimiento de la parálisis entre noviembre y diciembre. Como estos pacientes no tienen dolor, sólo fueron conscientes del problema cuando habían perdido la forma, la destreza y la fuerza de la mano”.
Tras un estudio de esos pacientes con pruebas específicas de conducción nerviosa, constató “un bloqueo agudo y casi completo de la actividad nerviosa en el canal cubital del codo, lo que en términos prácticos se asemejaba a una sección del nervio”.
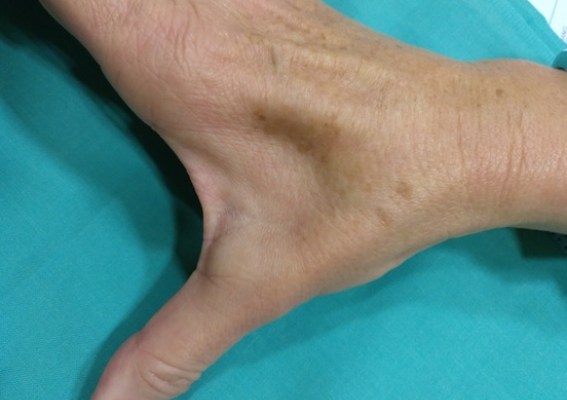
Dada esta rápida evolución y la comprobación del bloqueo casi completo de la actividad nerviosa mediante los estudios específicos, González del Pino optó por una cirugía de liberación de manera inmediata. “Ya he intervenido los primeros pacientes y los hallazgos son sorprendentes: el nervio discurre en el interior de una canal de por sí estrecho, pero pude ver que el ligamento que forma su pared se había retraído, endurecido y lo comprimía, como si hubiera sido estrangulado por una brida apretada al máximo. Se tenía la sensación de que en la zona de máxima compresión el nervio estaba vacío, como si no hubiera nada dentro: los grupos fasciculares que permiten la conducción nerviosa se encontraban extremadamente adelgazados, asemejando un reloj de arena“.
Tras la cirugía de descompresión y transposición del nervio, “incluyendo su vascularización a una zona bajo la musculatura flexo-pronadora de mejores características biomecánicas, en los primeros días los pacientes notaron mejora de la sensibilidad, coordinación de los movimientos finos de la mano y desaparición de las deformidades en garra de los dedos. Aunque no se puede presuponer el éxito a largo plazo, los resultados preliminares son extraordinariamente esperanzadores”, expone.
El especialista opina que probablemente otros colegas en el mundo habrán visto lesiones similares, si bien no hay nada publicado de forma explícita al respecto.
Lo llamativo de estos cuadros es “la velocidad con que se instaura el problema: parálisis producidas en 2-4 meses y no en 10-15 años, que es lo normal cuando el nervio cubital se atrapa en el codo. A pesar de no tener un conocimiento completo de estos problemas en los pacientes con covid-19, la lógica dice que cuanto antes se descomprima el nervio del túnel que lo estrangula, mayores son las posibilidades de recuperación, por eso la cirugía inmediata es, en mi opinión, la única forma de tratamiento: si no se hace rápidamente los músculos atrofiados degeneran, se fibrosan y pierden su capacidad de recuperación”.
Respecto a la evolución a largo plazo, evita hacer afirmaciones rotundas, “porque las toxinas del virus también dañan la fibra nerviosa, pero esta cirugía permite recuperar el riego sanguíneo del nervio y ayudar de forma evidente a su recuperación”.
Síndrome del túnel carpiano agudo
Además de las atrofias musculares debidas a parálisis cubital, González del Pino relaciona ciertos casos del síndrome del túnel carpiano agudo con el Covid, según ha podido observar en varias mujeres de mediana edad. “En este caso no se produce parálisis, sino un dolor repentino e hiperagudo, brusco y violento, que se manifiesta en los dedos pulgar, índice y corazón, y que se incrementa por la noche, impidiendo conciliar el sueño. Algunas ya tenían síntomas leves antes de contraer la infección y se agravaron bruscamente, y otras eran totalmente asintomáticas. En todos los casos el dolor les hace despertarse varias veces, obliga a levantarse, meter las manos en agua fría o caliente y moverlas para mitigar, sólo parcialmente, el dolor. Y así durante semanas. Los estudios de actividad nerviosa reflejaron igualmente afectación grave del nervio mediano a su paso por la muñeca. El denominador común en todas ellas es haber pasado la enfermedad en primavera o después del verano”.
De nuevo se imponía una cirugía inmediata de liberación del nervio, que “produjo la desaparición absoluta e inmediata del dolor y la recuperación completa de la sensibilidad en la primera semana”.
Causas múltiples
En cuanto a la etiopatogenia, González del Pino indica que es multifactorial. No hay apenas bibliografía explícita al respecto, explica, pero “extrapolando la existente a mis casos puedo sugerir como causa probable un proceso de desmielinización focal por inflamación de origen autoinmune, pero no puede descartarse un efecto neurotóxico directo del coronavirus sobre la fibra nerviosa. Determinados nervios (cubital, mediano y ciático-políteo externo) en determinadas zonas estrechas (canal cubital en el codo, túnel carpiano y cabeza del peroné) pudieran ser más susceptibles a fenómenos isquémicos secundarios a vasculitis (afectación endotelial secundaria a “tormenta de citoquinas”), reducción de la microcirculación nerviosa por microtromosis, neuropatía autoinmune o toxicidad directa sobre la fibra nerviosa. Tampoco se puede descartar la posibilidad de neuropatía desmielinizante inflamatoria (“estatus de hiperinflamación e hipercoagulabilidad”). Algunos autores han encontrado parálisis nerviosas de las extremidades por compresión mantenida secundarios a posición prona en pacientes en UVI por distress respiratorio agudo o por hematoma organizado secundario a anticoagulación o edema perineural residual. En estos casos los nervios más afectados son el cubital en el codo y el ciático poplíteo externo en la rodilla. Sin embargo ninguno de nuestros pacientes estuvo ingresado ni recibió tratamiento anticoagulante, por lo que la teoría posicional o hemorrágica puede descartarse”.
Por estos motivos, continúa, parece que la compresión (atrapamiento) se produce de novo en zonas susceptibles por sus especiales características anatómicas (canal cubital en el codo y túnel carpiano), aunque desencadenada por una causa aún no bien dilucidada y probablemente múltiple: respuesta hiperinmune con inflamación, isquemia nerviosa y neurotoxicidad directa a cualquiera de las estructuras nerviosas, vainas o contenido fascicular.
“El agravamiento de nuestros pacientes con síndrome del túnel carpiano también está en la base de estos hechos (edema intracanal y disminución de la microcirculación intraneural del nervio mediano), aunque no existen datos que puedan corroborar una u otra causa”.
Otros efectos músculo-esqueléticos
El cirujano alude a otros posibles efectos de la covid-19 en el sistema músculo-esquelético de los brazos. “He atendido pacientes con “codo de tenista” y diversas tendinitis de la muñeca, así como agravamiento de la enfermedad de Dupuytren”.
Ante los casos descritos, advierte a los pacientes de “que si se despiertan bruscamente con dolor y adormecimiento en las manos (sobre todo por la noche), y a aquellos que noten cómo adelgaza su mano y pierden la habilidad de uso, que acudan al especialista de Traumatología o a un experto en cirugía de la mano”.
Strep A and Tic Worsening: Final Word?

Exposure to Group A streptococcus (GAS) does not appear to worsen symptoms of Tourette syndrome and other chronic tic disorders (CTDs) in children and adolescents, new research suggests.
Investigators studied over 700 children and teenagers with CTDs, one third of whom also had attention deficit hyperactivity disorder (ADHD) and one third who had obsessive-compulsive disorder (OCD).
The youngsters were followed for an average of 16 months and evaluated at 4-month intervals to see if they were infected with GAS. Tic severity was monitored through telephone interviews, in-person visits, and parental reports.
A little less than half the children experienced worsening of tics during the study period, but the researchers found no association between these exacerbations and GAS exposure.
There was also no link between GAS and worsening OCD. However, researchers did find an association between GAS exposure and an increase in hyperactivity and impulsivity in patients with ADHD.
“This study does not support GAS exposures as contributing factors for tic exacerbations in children with CTD,” the authors note.
“Specific work-up or active management of GAS infections is unlikely to help modifying the course of tics in CTD and is therefore not recommended,” they conclude.
The study was published online February 10 in Neurology.https://6ca5e71ac1dd37b9cb45471ee9ba2603.safeframe.googlesyndication.com/safeframe/1-0-37/html/container.html
“Intense Debate”
The association between GAS and CTD stems from the description of Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal infection (PANDAS) — a condition that is now incorporated in the pediatric acute neuropsychiatric syndromes (PANS), the authors note. Tics constitute an “accompanying feature” of this condition.
However, neither population-based nor longitudinal clinical studies “could definitely establish if tic exacerbations in CTD are associated with GAS infections,” they note.
“The link between streptococcus and tics in children is still a matter of intense debate,” said study author Davide Martino, MD, PhD, director of the Movement Disorders Program at the University of Calgary, Calgary, Canada, in a press release.
“We wanted to look at that question, as well as a possible link between strep and behavioral symptoms like obsessive-compulsive disorder and attention deficit hyperactivity disorder,” he said.
The researchers followed 715 children with CTD (mean age 10.7 years, 76.8% male) who were drawn from 16 specialist clinics in nine countries. Almost all (90.8%) had a diagnosis of Tourette syndrome (TS); 31.7% had OCD and 36.1% had ADHD.
Participants received a throat swab at baseline, and of these, 8.4% tested positive for GAS.
Participants were evaluated over a 16- to 18-month period, consisting of:
- Face-to-face interviews and collection of throat swabs and serum at 4-month intervals
- Telephone interviews at 4-month intervals, which took place at 2 months between study visit
- Weekly diaries; parents were asked to indicate in the diary worsening of tics and focus on detecting the earliest possible tic exacerbation.
Beyond the regularly scheduled visits, parents were instructed to report by phone or email any noticeable increase in tic severity and then attend an in-person visit.
Tic exacerbations were defined as an increase of ≥ 6 points on the Yale Global Tic Severity Scale-Total Tic Severity Score (YGTSS-TTS), compared with the previous assessment.
OCD and ADHD symptoms were assessed according to the Yale-Brown Obsessive-Compulsive Scale and the parent-reported Swanson, Nolan, and Pelham-IV (SNAP-IV) questionnaire.
The researchers divided GAS exposures into 4 categories: new definite exposure; new possible exposure; ongoing definite exposure; and ongoing possible exposurehttps://6ca5e71ac1dd37b9cb45471ee9ba2603.safeframe.googlesyndication.com/safeframe/1-0-37/html/container.html
Unlikely Trigger
During the follow-up period, 43.1% (n = 308) of participants experienced tic exacerbations. Of these, 218 participants experienced one exacerbation, while 90 participants experienced two, three, or four exacerbations.
The researchers did not find a significant association between GAS exposure status and tic exacerbation.
Participants who did develop a GAS-associated exacerbation (n = 49) were younger at study exit (9.63 vs. 11.4 years, P < .0001) and were more likely to be male (46/49 vs 210/259, Fisher’s = .035), compared with participants who developed a non-GAS-associated tic exacerbation (n = 259).
Additional analyses were adjusted for sex, age at onset, exposure to psychotropic medications, exposures to antibiotics, geographical regions, and number of visits in the time interval of interest. These analyses continued to yield no significant association between new or ongoing concurrent GAS exposure episodes and tic exacerbation events.
Of the children in the study, 103 had a positive throat swab, indicating a new definite GAS exposure, whereas 46 had a positive throat swab indicating an ongoing definite exposure (n = 149 visits). Of these visits, only 20 corresponded to tic exacerbations.
There was also no association between GAS exposure and OCD symptom severity. However, it was associated with longitudinal changes (between 17% and 21%, depending on GAS exposure definition) in the severity of hyperactivity-impulsivity symptoms in children with ADHD.
“It is known that immune activation may concur with tic severity in youth with CTDs, and that psychosocial stress levels may predict short-term future tic severity in these patients,” the authors write.
“Our findings suggest that GAS is unlikely to be the main trigger for immune activation in these patients,” they add.
Brick or Cornerstone?
Commenting on the study for Medscape Medical News, Margo Thienemann, MD, clinical professor of psychiatry, Stanford University School of Health, Stanford, California, said that in the clinic population they treat, GAS, other pathogens, and other stresses can “each be associated with PANS symptom exacerbations.”
However, these “would not be likely to cause PANS symptoms exacerbations in the vast majority of individuals, only individuals with genetic backgrounds and immunologic dysfunctions creating susceptibility,” said Thienemann, who also directs the Pediatric Acute-Onset Neuropsychiatric Syndrome (PANS) Clinic at Stanford Children’s Health. She was not involved with the study.
In an accompanying editorial, Andrea Cavanna, MD, PhD, honorary reader in neuropsychiatry, Birmingham Medical School, UK, and Keith Coffman, MD, director, Tourette Syndrome Center of Excellence, Children’s Mercy Hospital, Kansas City, Missouri, suggest that perhaps the “interaction of psychosocial stress and GAS infections contributes more to tic exacerbation than psychosocial stress alone.”
“Time will tell whether this study stands as another brick — a cornerstone?— in the wall that separates streptococcus from tics,” they write.
The study was supported by the European Union’s Seventh Framework Program. Martino has received honoraria for lecturing from the Movement Disorders Society, Tourette Syndrome Association of America, and Dystonia Medical Research Foundation Canada; research funding support from Dystonia Medical Research Foundation Canada, the University of Calgary, the Michael P Smith Family, the Owerko Foundation, Ipsen Corporate, the Parkinson Association of Alberta, and the Canadian Institutes for Health Research; and royalties from Springer-Verlag. The other authors’ disclosures are listed in the original article. Cavanna, Coffman, and Thienemann have disclosed no relevant financial relationships.
Neurology. Published online February 10, 2021. Abstract, Editorial
Medscape Medical News © 2021
Cite this: Strep A and Tic Worsening: Final Word? – Medscape – Feb 12, 2021.
Prostate Drugs Tied to Lower Risk for Parkinson’s

Certain drugs currently used to treat benign prostatic hyperplasia (BPH) may provide neuroprotection and delay or prevent the onset of Parkinson’s disease, new research suggests.
Dr Jacob Simmering
“If giving someone terazosin or similar medications truly reduces their risk of disease, these results could have significant clinical implications for neurologists,” Jacob E. Simmering, PhD, assistant professor of internal medicine at the University of Iowa in Iowa City, told Medscape Medical News.
There are few reliable neuroprotective treatments for Parkinson’s disease, he said. “We can manage some of the symptoms, but we can’t stop it from progressing. If a randomized trial finds the same result, this will provide a new option to slow progression of Parkinson’s disease,” Simmering said.
The pathogenesis of Parkinson’s disease is heterogeneous, however, and not all patients may benefit from glycolysis-enhancing drugs, the investigators note. Future research will be needed to identify potential candidates for this treatment, and clarify the effects of these drugs, they write.
The findings were published online February 1 in JAMA Neurology.
Time-Dependent Effects
The major risk factor for Parkinson’s disease is age, which is associated with impaired energy metabolism. Glycolysis is decreased among patients with Parkinson’s, yet impaired energy metabolism has not been investigated widely as a pathogenic factor in the disease, the authors write.
Studies have indicated that terazosin increases the activity of an enzyme important in glycolysis. Doxazosin and alfuzosin have a similar mechanism of action and enhance energy metabolism. Tamsulosin, a structurally unrelated drug, has the same mechanism of action as the other three drugs, but does not enhance energy metabolism.
In this report, the researchers investigated the hypothesis that patients who received therapy with terazosin, doxazosin, or alfuzosin would have a lower risk of developing Parkinson’s than patients receiving tamsulosin. To do that, they used healthcare utilization data from Denmark and the United States, including the Danish National Prescription Registry, the Danish National Patient Registry, the Danish Civil Registration System, and the Truven Health Analytics MarketScan database.
The investigators searched the records for patients who filled prescriptions for any of the four drugs of interest. They excluded any patients who developed Parkinson’s within 1 year of starting medication. Because use of these drugs is rare among women, they included only men in their analysis.
They looked at patient outcomes beginning at one year after the initiation of treatment. They also required patients to fill at least two prescriptions before the beginning of follow-up. Patients who switched from tamsulosin to any of the other drugs, or vice versa, were excluded from analysis.
The investigators used propensity score matching to ensure that patients in the tamsulosin and terazosin/doxazosin/alfuzosin groups were similar in terms of their other potential risk factors. The primary outcome was the development of Parkinson’s disease.
They identified 52,365 propensity score-matched pairs in the Danish registries and 94,883 pairs in the Truven database. The mean age was 67.9 years in the Danish registries and 63.8 years in the Truven database, and follow-up was approximately 5 years and 3 years respectively. Baseline covariates were well balanced between cohorts.
Among Danish patients, those who took terazosin, doxazosin, or alfuzosin had a lower risk of developing Parkinson’s vs those who took tamsulosin (hazard ratio [HR], 0.88). Similarly, patients in the Truven database who took terazosin, doxazosin, or alfuzosin had a lower risk of developing Parkinson’s than those who took tamsulosin (HR, 0.63).
In both cohorts, the risk for Parkinson’s among patients receiving terazosin, doxazosin, or alfuzosin, compared with those receiving tamsulosin, decreased with increasing numbers of prescriptions filled. Long-term treatment with any of the three glycolysis-enhancing drugs was associated with greater risk reduction in the Danish (HR, 0.79) and in the Truven (HR, 0.46) cohorts, vs tamsulosin.
Differences in case definitions, which may reflect how Parkinson’s disease was managed, complicate comparisons between the Danish and Truven cohorts, said Simmering. Another challenge is the source of the data.
“The Truven data set was derived from insurance claims from people with private insurance or Medicare supplemental plans,” he told Medscape Medical News. “This group is quite large but may not be representative of everyone in the United States. We would also only be able to follow people while they were on one insurance plan. If they switched coverage to a company that doesn’t contribute data, we would lose them.”
The Danish database, however, includes all residents of Denmark. Only people who left the country were lost to follow-up.
The results support the hypothesis that increasing energy in cells slows disease progression, Simmering added. “There are a few conditions, mostly REM sleep disorders, that are associated with future diagnosis of Parkinson’s disease. Right now, we don’t have anything to offer people at elevated risk of Parkinson’s disease that might prevent the disease. If a controlled trial finds that terazosin slows or prevents Parkinson’s disease, we would have something truly protective to offer these patients.”
Biomarker Needed
Dr Alberto Espay
Commenting on the results, Alberto J. Espay, MD, MSc, professor of neurology at University of Cincinnati Academic Health Center, Cincinnati, Ohio, was cautious. “These findings are of unclear applicability to any particular patient without a biomarker for a deficit of glycolysis that these drugs are presumed to affect,” Espay told Medscape Medical News. “Hence, there is no feasible or warranted change in practice as a result of this study.”
Pathogenic mechanisms are heterogeneous among patients with Parkinson’s disease, Espay added. “We will need to understand who among the large biological universe of Parkinson’s patients may have impaired energy metabolism as a pathogenic mechanism to be selected for a future clinical trial evaluating terazosin, doxazosin, or alfuzosin as a potential disease-modifying intervention.”
Parkinson’s is not one disease, but a group of disorders with unique biological abnormalities, said Espay. “We know so much about ‘Parkinson’s disease’ and next to nothing about the biology of individuals with Parkinson’s disease.”
This situation has enabled the development of symptomatic treatments, such as dopaminergic therapies, but failed to yield disease-modifying treatments, he said.
The University of Iowa contributed funds for this study. Simmering has received pilot funding from the University of Iowa Institute for Clinical and Translational Science. He had no conflicts of interest to disclose. Espay has disclosed no relevant financial relationships.
JAMA Neurol. Published online February 1, 2021. Full text
Medscape Medical News © 2021
Cite this: Prostate Drugs Tied to Lower Risk for Parkinson’s – Medscape – Feb 09, 2021.
Las siestas vespertinas están relacionadas con una mejor cognición en adultos mayores

Las siestas por la tarde de los adultos mayores se vinculan con una mejor memoria y mejores habilidades del lenguaje, sugiere una nueva investigación.[1]
Un estudio aleatorizado con más de 2000 adultos mayores en China mostró que aquellos que tomaron siestas por la tarde que duraron menos de 2 horas obtuvieron puntuaciones significativamente mejores en las medidas de función cognitiva que sus contrapartes que no tomaron siestas.
Los puntajes totales y los puntajes de orientación y función del lenguaje en el Mini-examen del estado mental (MMSE) fueron significativamente más altos en el grupo de siesta, al igual que los puntajes de orientación en la versión de Beijing de la Evaluación cognitiva de Montreal (MoCA). Otras pruebas también mostraron diferencias significativas entre los grupos en la fluidez del lenguaje y la extensión de los dígitos.
Curiosamente, un subanálisis mostró que entre los voluntarios que se sometieron a pruebas de lípidos en sangre, los que tomaron una siesta tenían niveles más altos de triglicéridos. No hubo diferencias entre los grupos en los niveles de colesterol, lipoproteínas de baja densidad (LDL) o lipoproteínas de alta densidad (HDL).
El autor para correspondencia Dr. Lin Sun del Alzheimer’s Disease and Related Disorders Center y del Departamento de Geriatría y Psiquiatría en el Shanghai Mental Health Center, en Shanghái, China, señaló que las siestas parecen ser muy importantes para esta población.
“Este estudio encontró que una siesta adecuada es buena para preservar la función cognitiva, por lo que alentamos a los adultos mayores a tomar una siesta adecuada”, dijo a Medscape Noticias Médicas el Dr. Sun, que también está afiliado a la Facultad de Medicina de la Shanghai Jiao Tong University.
Los hallazgos se publicaron en versión electrónica el 25 de enero en General Psychiatry.
Hallazgos contradictorios
Los estudios anteriores que han examinado la función cognitiva y la siesta han tenido resultados contradictorios, algunos muestran resultados positivos y otros sugieren lo contrario. Para el estudio actual, los investigadores buscaron examinar la asociación en una población donde prevalece una “cultura de la siesta”.
“La siesta es muy común en los chinos, incluso es una característica nacional, y los adultos mayores tienen una mayor frecuencia de siestas”, dijo el Dr. Sun. Entonces, “recopilamos información relevante en una gran encuesta y los resultados del análisis fueron interesantes”.
Los investigadores analizaron datos de una gran encuesta epidemiológica nacional e incluyeron a 2.214 participantes de 55 años o más. De estos, 1.534 (69,3%) informaron que dormían una siesta. La siesta se definió como la participación en “periodos de al menos cinco minutos consecutivos calificados como sueño (inactividad) después del almuerzo, fuera del horario principal de sueño”.
Los participantes también informaron sobre la frecuencia y se recopilaron datos demográficos.
Los investigadores no encontraron diferencias significativas entre los que dormían y los que no dormían una siesta, con respecto al sexo (masculino 58,4%, frente a femenino 59,7%) y edad promedio (71 frente a 70 años), años de educación (7,37 frente a 6,95) y horas de sueño nocturno (6,54 frente a 6,61 horas). Tampoco hubo diferencias significativas entre los grupos entre los participantes que fumaban, bebían alcohol o que tenían hipertensión o diabetes.
Los criterios de exclusión incluyeron antecedentes de enfermedad mental o disfunción cognitiva, ceguera, sordera o cualquier otro trastorno físico importante.
Además del MMSE y la versión de Beijing del MoCA, todos los participantes fueron evaluados utilizando la versión china del Neuropsychological Test Battery (NTB). Un subgrupo de 739 voluntarios también se sometió a pruebas de lípidos en sangre (personas que dormían siestas: n = 428).
¿Mediador inflamatorio?
Los resultados mostraron que el grupo que duerme la siesta tenía una puntuación total media del MMSE significativamente mayor que el grupo que no tomaba la siesta (25,3 frente a 24,56; p = 0,003), así como una mayor orientación (9,28 frente a 9,01; p < 0,001) y lenguaje (7,27 frente a 7,06; p = 0,02) en las puntuaciones del MMSE.https://f2aa3c66d1ae67c0137372eeeccf68f0.safeframe.googlesyndication.com/safeframe/1-0-37/html/container.html
Además, los que duermen la siesta obtuvieron puntuaciones más altas de orientación en el MoCA (5,55 frente a 5,41; p = 0,006) y una mayor recolección de dígitos (3,24 frente a 12,48; p = 0,009) y fluidez del lenguaje (24,35 frente a 23,23; p = 0,02) en el NTB.
En el subgrupo que se sometió a la prueba de lípidos, los que dormían siestas también mostraron niveles más altos de triglicéridos que los que no tomaron siestas (1,80 frente a 1,75 mmol/l; p = 0,001).
“Los resultados demuestran que las siestas de la tarde estaban relacionadas con una mejor función cognitiva en la población china de edad mayor”, escriben los investigadores.
Con respecto a los posibles mecanismos que podrían estar ocurriendo, los investigadores señalan que la “actividad de las citocinas inflamatorias juega un papel importante en los trastornos del sueño”.
La siesta “se cree que es una respuesta evolucionada a la inflamación”, y aquellos “con niveles más altos de inflamación también duermen la siesta con más frecuencia”, añaden los investigadores.
Las limitaciones del estudio incluyen su diseño transversal y que no puede mostrar causalidad directa.
“Sabemos que el sueño es importante para la salud cognitiva desde una variedad de ángulos diferentes, pero el papel de las siestas todavía está en debate”, dijo Sexton, quien no participó en la investigación actual.
Señaló que, aunque este estudio demostró que las siestas son benéficas, estudios previos las han vinculado con un empeoramiento del deterioro cognitivo en el futuro.
“Este es un estudio importante… pero no nos ha llevado a la respuesta definitiva ni al entendimiento definitivo todavía”, dijo Sexton.
Señaló que el estudio destacó algunos factores importantes que deberían tenerse en cuenta, incluida la forma en que se miden las siestas. También señaló que la duración de la siesta puede ser importante.
El estudio actual limitó las siestas a no más de 2 horas, mientras que la mayoría de los estudios anteriores incluyeron siestas más largas. “Por lo tanto, podría ser que estas siestas más largas estén asociadas con un mayor deterioro cognitivo”, anotó Sexton.
Otro punto importante, dijo, se refiere al contexto de la siesta. Por ejemplo, una siesta puede ser compensatoria y beneficiosa si ocurre después de una mala noche de sueño. “Pero si duermes bien por la noche y aún necesitas tomar largas siestas por la tarde, eso podría ser más una señal de advertencia”, agregó.
Cuando se le preguntó si los hallazgos del estudio se pueden generalizar a la población de Estados Unidos, Sexton señaló que cerca de 70% de los participantes informaron haber tomado algún tipo de siesta, que puede ser alta en comparación con otros estudios.
“La cultura de la siesta varía de una región a otra, lo que puede limitar la traducción de los hallazgos. Por eso es tan importante que este tipo de investigación se realice en diferentes países, para que podamos comprender mejor qué factores son comunes y qué factores podrían estar limitados por factores regionales”, concluyó Sexton.
El estudio fue financiado por subvenciones del Shanghai Mental Health Center, Shanghai Science and Technology Commission, National Natural Science Foundation of China y Shanghai Jiao University School of Medicine . Los autores del estudio y Sexton han informado no tener ningún conflicto de interés económico pertinente.
Medscape Noticias Médicas © 2021
Citar este artículo: Las siestas vespertinas están relacionadas con una mejor cognición en adultos mayores – Medscape – 5 de feb de 2021.
Thunderclap Headache Often Mismanaged in the ED

More than 50% of patients who present to the emergency department (ED) with thunderclap headache (TCH), which often signals an underlying serious or life-threatening condition, are inadequately managed, new research shows.
Dr David García-Azorín
“Most of the associated conditions are secondary disorders that might threaten patients’ lives or cause persistent disability, so the management should be as precise and efficient as possible. If neurologists say that ‘time is brain,’ then thunderclap should be managed as promptly as code stroke,” study investigator David García-Azorín, MD, a neurologist at Hospital Clínico Universitario de Valladolid, in Valladolid, Spain, told Medscape Medical News.
The study was published online January 7 in Cephalalgia.
A Potential Medical Emergency
TCH is primarily associated with subarachnoid hemorrhage (SAH), but there are many other disorders, including cerebral venous sinus thrombosis, intracranial infection, increased intracranial pressure, and reversible cerebral vasoconstriction syndrome (RCVS), that present as TCH.
To evaluate how often clinicians rule out secondary headache disorders for patients with TCH, the investigators examined electronic health records for consecutive patients who visited the ED at a single center between January 2012 and July 2014.https://7da40b413b6eb19a90c79e3961a14f93.safeframe.googlesyndication.com/safeframe/1-0-37/html/container.html
Eligible patients had pain of severe intensity that reached its peak within 1 minute of headache onset, with the headache lasting for at least 5 minutes. Patients who had recently experienced traumatic injury to the head or neck were excluded from the study.
The study’s primary endpoint was how often a secondary cause of TCH was adequately ruled and whether patient assessment was in line with International Classification of Headache Disorders (ICHD) recommendations.
ICHD guidance for ruling out secondary causes of TCH include cranial CT, lumbar puncture, CT angiography (CTA), magnetic resonance angiography (MRA), digital subtraction angiography, and MRI.
The researchers also determined the proportion of patients who were examined by a neurologist, who were admitted to hospital, and who were discharged with no further examination.
Secondary Causes Common
Of 2132 patients who visited the ED for headache, 71 patients were eligible, and 29 were excluded. The final analysis included 42 patients. The mean age of the study population was approximately 43 years, and 24 patients (57.1%) were women. Twenty patients (47.6%) had a prior history of headache.
Secondary causes of TCH were insufficiently ruled out for 22 (52.4%) patients with TCH. The most commonly omitted exams were vascular evaluation (38.1% of the entire sample, 72.7% of patients with incomplete workup), cerebrospinal fluid (CSF) evaluation (21.4% of the entire sample, 40.9% of patients with incomplete workup), and MRI (16.7% of the entire sample, 31.8% of patients with incomplete workup).
Multiple examinations were omitted for six patients (27.3%). Eight patients (19.0%) received a diagnosis of primary headache, even though their assessment was incomplete.
All patients underwent cranial CT as an initial investigation. A total of 18 patients (52.9%) underwent lumbar puncture, 10 (29.4%) underwent CTA, and two (5.8%) underwent MRI. A total of 28 (66.7%) patients were evaluated by a neurologist. Four patients (11.7%) did not undergo any further examination.
The investigators found a secondary cause for TCH in 16 of the 20 patients whose assessment was adequate. Of these, eight had SAH, two had RCVS, and two had intracerebral hemorrhage secondary to arteriovenous malformation rupture.
The remaining causes were amyloid angiopathy–associated intracerebral hemorrhage, CSF low-pressure headache, unruptured intracranial aneurysm, and pituitary space-occupying lesion with signs of bleeding on MRI.https://7da40b413b6eb19a90c79e3961a14f93.safeframe.googlesyndication.com/safeframe/1-0-37/html/container.html
Primary headache was diagnosed in the four patients for whom a secondary cause of TCH was adequately ruled out. These diagnoses included primary headache associated with sexual activity (n = 2), primary TCH (n = 1), and primary cough headache (n = 1).
The median time from patient arrival to CT request was 68.0 minutes. The median time from CT request to CT was 66.0 minutes. Imaging was completed within an hour after request in 47.6% of patients and within an hour of patient arrival in 42.8% patients.
The time between patient arrival and lumbar puncture was 262.2 minutes, and the time between the first imaging and lumbar puncture was 83.5 minutes.
Despite the many potential causes of TCH, many clinicians believe that results of imaging and CSF analysis can rule them out, said García-Azorín.
A clear case of TCH is a “red flag” and requires more sophisticated imaging and further investigation, said García-Azorín.
“In these cases, imaging should be requested as urgently as we do in code stroke, and in the case of normal study, the craniocervical arterial and venous system should be directly evaluated in the same study. With this approach, many secondary causes could be detected, with a huge impact on the prognosis of many of them,” he said.
Some patients were discharged before their studies were completed, so the frequency of secondary causes may be underrepresented in the current investigation, García-Azorín noted.
“To properly describe all the causes of thunderclap headache, increasing the sample size and combining our efforts with other international centers could give us better insights on the causes and potential consequences of this intriguing syndrome,” he said.
Diagnosis Is Key
Dr Stewart Tepper
The study reveals that EDs are not following through on the recommended workup for the management of TCH, said Tepper. He noted that the ICHD recommendations need to be followed “as quickly as possible” for optimal screening and care. “We cannot either defer workup or elect not to go through workup,” he said.
Vascular imaging and MRI can reveal secondary causes of headache, so performing only CT and spinal tap is inadequate, he said. If results of all four of these tests are negative, vascular imaging should be repeated 1 month later to screen for RCVS.
“Getting the diagnosis is key. You need to know why somebody presents with a thunderclap. They need to have this study in every emergency room in the country, because the number of cases they had was quite high and…more than 50% were not adequately worked up,” said Tepper.
The study’s strengths, he added, include a clear statement of its objectives, the number of patients that the investigators considered for the study, and the assessment of the length of time it took for patients to undergo various tests, said Tepper.
The study’s potential limitations include its retrospective design, that fact that it was a single-center study, and the small size of the final sample. A prospective study potentially could provide more meaningful results, Tepper noted.
“I think we have our work cut out for us,” he added. “Headache providers and neurology providers who counsel emergency providers need to talk them through this, need to teach them, need to have them adopt this. We also have to teach the neurologists who consulted, because they didn’t get it right all the time either,” he concluded.
The study was conducted without outside funding. García-Azorín and Tepper have disclosed no relevant financial relationships.
Cephalalgia. Published online January 7, 2021. Abstract
Medscape Medical News © 2021
Cite this: Thunderclap Headache Often Mismanaged in the ED – Medscape – Feb 04, 2021.
“Coronasomnia”: el insomnio generalizado por coronavirus y la automedicación preocupan a expertos en sueño

Entre las innumerables pérdidas sufridas por millones de personas en todo el mundo durante la pandemia de COVID-19, la pérdida de sueño puede ser la más generalizada, con consecuencias negativas potencialmente prolongadas para la salud física, mental y emocional, según observaron investigadores del sueño.[1]
Los resultados de múltiples estudios y encuestas que se han llevado a cabo durante la pandemia muestran que una mayoría de individuos refiere cambios clínicamente significativos en la calidad, los patrones y las alteraciones del sueño.
Por ejemplo, una encuesta internacional representativa realizada desde finales de marzo hasta finales de abril de 2020 reveló que entre más de 3.000 informantes de 49 países, 58 de cada 100 refirieron insatisfacción con su sueño y 40% informó disminución de la calidad del sueño durante la pandemia, en comparación con el sueño previo a la COVID-19, de acuerdo con Uri Mandelkorn, de la Clínica de Sueño Natural en Jerusalén, Israel, y sus colaboradores.
“En particular, esta investigación plantea la necesidad de evaluar el empeoramiento de los patrones de sueño y utilizar ayuda para dormir en las poblaciones más susceptibles identificadas en este estudio, es decir, mujeres y personas con modos de vida inseguros o que están sujetas a cuarentena estricta. El personal sanitario debe prestar especial atención a los problemas físicos y psicológicos que este repunte en las alteraciones del sueño puede ocasionar”, escribieron. El estudio fue publicado en Journal of Clinical Sleep Medicine.
Dormir, más o menos
Un coautor de este estudio, el Dr. David Gozal, neumólogo pediatra y especialista en medicina del sueño en la University of Missouri en Columbia, Estados Unidos, dijo que la pandemia ha tenido efectos paradójicos en los patrones de sueño de muchas personas.
“Al principio, en las fases iniciales del confinamiento por COVID-19, la mayoría de las personas cuyos trabajos no se vieron afectados y que no los perdieron, quienes no tenían la ansiedad por quedarse sin trabajo y con problemas económicos, pero que ahora se están quedando en casa, hubo en realidad un beneficio. Las personas comenzaron a informar que dormían más, y lo que es más importante, sueños más vívidos y cosas de esa naturaleza”, comentó.
“Pero a medida que avanzó el confinamiento vimos progresivamente que cada vez más personas tenían dificultades para dormir y mantenerse dormidos, utilizando más fármacos, como hipnóticos para inducir el sueño, y vimos un incremento de 20% en el consumo general de pastillas para dormir”, dijo.
Se observaron resultados similares en una encuesta representativa de 843 adultos de Reino Unido, que demostró que casi 70% de los participantes refirió un cambio en los patrones de sueño; solo 45% informó tener un sueño reparador y 46% reportó dormir más durante el confinamiento que antes de este.[2] Dos tercios de los informantes notificaron que la pandemia afectó su salud mental, y una cuarta parte informó aumento del consumo de alcohol durante el confinamiento. Aquellos con COVID-19 sospechada refirieron tener más pesadillas y ritmos de sueño anormales.
Es posible que los efectos de la COVID-19 sobre el sueño puedan prolongarse mucho después de que se haya resuelto la infección en sí, señalan los resultados de un estudio de cohortes en China. Como se informó en The Lancet, de 1.655 pacientes dados de alta del hospital Jin Yin-tan, en Wuhan, China, 26% refirió alteraciones del sueño seis meses después de la COVID-19 aguda.[3]
Automedicación
De los 5.525 canadienses encuestados del 3 de abril al 24 de junio de 2020, gran proporción refirió el uso de auxiliares farmacológicos para dormir, señaló Tetyana Kendzerska, Ph. D., profesora asistente de medicina en la sección de respirología en la University of Ottawa, en Ottawa, Canadá.[4]
“En el periodo en que se llevó a cabo la encuesta, 27% de los participantes refirió tomar auxiliares para el sueño (prescritos o de venta libre); de toda la muestra, 8% de participantes reportó aumento de la frecuencia de uso de fármacos para dormir durante la pandemia, en comparación con el periodo previo a la misma”, declaró.
Muchas personas recurren a la automedicación con preparados de venta libre como melatonina y formulaciones para el alivio del dolor nocturno que contienen difenhidramina, un antihistamínico de primera generación con propiedades sedantes, señaló el Dr. Kannan Ramar, especialista en cuidados intensivos, enfermedades pulmonares y medicina del sueño en la Mayo Clinic, en Rochester, Estados Unidos, y actual presidente de la American Academy of Sleep Medicine.
“Cuando las personas se automedican por lo que consideran dificultad para dormir, existe la inquietud de que aun cuando se haya establecido un diagnóstico de insomnio podría haber otro trastorno del sueño persistente que pueda no haberse diagnosticado, y que pudiera estar causando el problema de insomnio”, destacó.
“Por ejemplo, la apnea obstructiva del sueño podría hacer que las personas despertaran por la noche e incluso contribuir a dificultades para quedarse dormido en primera instancia. Así que medicarse para tratar algo sin un diagnóstico conocido puede dejar un trastorno de sueño subyacente sin tratar, lo que no ayudará al paciente a corto ni a largo plazo”, indicó el Dr. Ramar.
Causas de inquietud
“En el caso de las personas que tienen COVID-19, hemos visto la aparición de muchos problemas de sueño. Esos no se comunicaron en el presente estudio, sino en los estudios clínicos y subsiguientes publicados en otros lugares”, dijo el Dr. Gozal.
“Entre las personas que padecieron COVID-19, incluso aquellas que supuestamente evolucionaron muy bien y que prácticamente estaban asintomáticas o tal vez tuvieron solo cefalea o fiebre pero que no necesitaron ir al hospital, muchas de ellas refirieron insomnio excesivo por un periodo prolongado, y dormían 2 o 3 horas más cada noche. O se notificó lo opuesto: quienes después de restablecerse informaron que no podían dormir; dormían 4 o 5 horas cuando normalmente duermen 7 u 8”, indicó.
No está claro con base en los datos actuales si el repunte notificado en los problemas de sueño también está relacionado.
El Dr. Gozal añadió que el insomnio en la época de COVID-19 podría atribuirse a diversos factores, como menos exposición diaria a la luz natural por parte de personas que se refugian dentro de casa, estrés relacionado con preocupaciones económicas o de salud, depresión u otros factores psicológicos.
Sin embargo, también es posible que los cambios fisiológicos relacionados con COVID-19 pudieran contribuir a trastornos del sueño, dijo, señalando un estudio reciente en Journal of Experimental Medicine que muestra que el SARS-CoV-2, el virus que produce COVID-19, puede unirse a las neuronas y causar cambios metabólicos en las células infectadas y en las vecinas.[5]
“Supongo que parte de ello está relacionado más con los efectos sobre la conducta; las personas desarrollan depresión, cambios en el estado de ánimo, ansiedad, etcétera, y todos estos pueden traducirse en dificultades con el sueño”, puntualizó.
“Podría ser que en algunos casos ―no con mucha frecuencia― el virus afecte áreas que controlan el sueño en nuestro cerebro y que, por tanto, veamos demasiado o muy poco sueño, y cómo distinguir entre todos estos es un área que sin duda necesita explorarse, en particular en vista del hallazgo de que el virus puede unirse a las células del cerebro e inducir problemas sustanciales en las mismas”.
Inmunidad afectada
Está bien documentado que además de ser, como lo llamó Shakespeare, “el bálsamo de las almas heridas”, el sueño tiene una función importante por cuanto brinda apoyo al sistema inmunitario.
“El sueño y la inmunidad van de la mano. Cuando las personas duermen bien se intensifica su sistema inmunitario. Sabemos que se dispone de buenos datos derivados de las vacunaciones contra hepatitis A y hepatitis B, y recientemente de la vacunación contra la influenza, que si las personas duermen lo suficiente antes y después de recibir la vacuna, su probabilidad de generar respuesta inmunitaria a esta vacuna en particular tiende a aumentar”, agregó el Dr. Ramar.
Es adecuado suponer que lo mismo sería aplicable para las vacunas contra COVID-19, pero esto aún no se ha demostrado, añadió.
“Sabemos, por los estudios previos, que los problemas de sueño persistentes pueden volver a las personas más susceptibles a infección o alterar su restablecimiento; aún no hay estudios, creo, desde la perspectiva de la COVID-19. En nuestro ensayo encontramos que, entre otros factores, alguna enfermedad crónica se asociaba con nuevas dificultades para dormir durante la pandemia. No analizamos por separado si las dificultades de sueño se asociaban con la COVID-19 o sus síntomas, pero es una excelente pregunta de abordar con los datos longitudinales con que contamos”, indicó Kendzerska.
¿Qué hacer?
Los tres expertos en sueño a los que se contactó para este artículo estuvieron de acuerdo que en el caso de pacientes con insomnio, mitigar el estrés con técnicas de relajación o terapia cognitiva conductual es más útil que con medicación.
“Los fármacos, incluso los de venta libre, tienen efectos secundarios, y si una persona toma un fármaco que contiene estimulantes, como seudoefedrina en combinaciones de antihistamínicos, esto puede contribuir a cualesquiera trastornos del sueño subyacentes o exacerbarlos”, dijo el Dr. Ramar.
Kendzerska recomendó reservar fármacos como melatonina, un fármaco cronobiótico, para pacientes con trastornos del sueño relacionados con problemas del ritmo circadiano, como un retraso en la fase del sueño. El tratamiento complementario a corto plazo mediante hipnóticos, como zolpidem, eszopiclona o zaleplon, se ha de utilizar solo como último recurso, destacó.
Los especialistas en medicina del sueño recomiendan una buena higiene de sueño como el mejor medio de obtener un sueño reparador, que incluya horarios regulares de sueño y vigilia, exposición limitada a noticias estresantes (incluidas las noticias sobre COVID-19), disminución del consumo de alcohol y estimulantes como café o bebidas con cafeína, evitar el uso de dispositivos electrónicos en la cama o cerca de la hora de acostarse, y un modo de vida sano, que incluya dieta y ejercicio.
Tampoco ven con buenos ojos la automedicación con pastillas para dormir de venta libre, porque esos productos no pueden resolver el problema subyacente, como se señaló antes.
“Asimismo, es previsible que haya más personas que puedan preferir orientación profesional para reducir gradualmente los fármacos para dormir iniciados o que se aumentaron durante la pandemia. Aunque algunos de estos problemas de sueño pueden ser transitorios, debería ser alta prioridad asegurarse de que no se conviertan en trastornos de sueño crónicos”, escribieron Kendzerska y sus colaboradores.
Caminos para la investigación
Si hay algo que quita el sueño a los especialistas, es la falta de datos o evidencia para orientar la atención clínica y la investigación. El Dr. Gozal resaltó que todavía se sabe poco acerca de los posibles efectos de COVID-19 sobre el sistema nervioso central, y dijo que debería ser un foco importante de investigación sobre el todavía nuevo coronavirus.
“Lo que ocurre después de COVID-19 y cómo podría afectar la recuperación subsiguiente, es una gran interrogante, y no creo que tengamos buenos datos al respecto. Lo que sabemos es que los pacientes desarrollan los síntomas de fatiga, alteraciones del sueño, incluso fiebre persistente, y lamentablemente, esto puede persistir por un periodo prolongado incluso en pacientes que por lo demás se han restablecido de COVID-19. Sabemos que si no se trata eso desde una perspectiva del trastorno del sueño puede exacerbar sus síntomas diarios, y es ahí donde recomendaría firmemente que buscaran ayuda de un médico especialista en sueño o, si tienen otros síntomas además del insomnio, por lo menos de un médico de atención primaria”.
Este artículo fue originalmente publicado en MDedge.com, parte de la Red Profesional de Medscape.
Medscape Noticias Médicas © 2021 WebMD, LLC
Citar este artículo: “Coronasomnia”: el insomnio generalizado por coronavirus y la automedicación preocupan a expertos en sueño – Medscape – 3 de feb de 2021.
#Cefaleia por exercício físico

A cefaleia por exercício é uma das várias síndromes de cefaleia relativamente raras que necessitam de uma avaliação médica cuidadosa. Ela pode ocorrer como um sintoma de outro distúrbio (cefaleia secundária) ou como um distúrbio primário sem uma anormalidade subjacente identificável (cefaleia primária ao exercício).
As anormalidades subjacentes mais comumente associadas à cefaleia por exercício incluem:
- Lesão traumática anterior;
- Lesões com efeito de massa supratentorial e fossa posterior;
- Anormalidades vasculares, como aneurisma cerebral ou malformação arteriovenosa;
- Hemorragia intracraniana.
Outros tipos de cefaleia paroxística que podem ser desencadeados por esforço incluem feocromocitoma, obstrução intermitente do fluxo do líquido cefalorraquidiano, causada por um cisto coloide de terceiro ventrículo por exemplo e malformação de Chiari tipo I. Importante lembrar que a cefaleia cardiogênica pode ser desencadeada por esforço em pacientes com fatores de risco para doença coronariana, incluindo aqueles sem dor torácica ou evidência de isquemia no eletrocardiograma (ECG). Sempre bom lembrar também que o exercício é um gatilho bastante comum em alguns pacientes com enxaqueca típica.
As características definidoras da cefaleia primária do exercício vêm de um número relativamente pequeno de séries de casos. É caracterizada por episódios de cefaleia bilateral, pulsátil, latejante, persistem de cinco minutos a 48 horas e são desencadeados por exercícios físicos. Geralmente não estão associadas a náuseas ou vômitos.
O diagnóstico de cefaleia primária do exercício, de acordo com a Classificação Internacional de Distúrbios de Cefaleia, 3ª edição (ICHD-3), requer o cumprimento de todos os seguintes critérios:
- Pelo menos dois episódios de cefaleia;
- Provocado e ocorrendo apenas durante ou após exercícios físicos extenuantes;
- Com duração de < 48 horas;
- Não melhor explicado por outro diagnóstico ICHD-3.
Mensagem final
Pacientes com cefaleia de exercício “nova” ou nunca avaliada devem passar por uma avaliação com especialista. Um estudo neurovascular muitas vezes se faz necessário, para descartar anormalidades vasculares ou outras causas estruturais, particularmente: hemorragia subaracnoide, dissecção arterial cerebral ou cervical e síndrome de vasoconstrição cerebral reversível. A necessidade de tais investigações aumenta quando os episódios de cefaleia aparecem após os 40 anos de idade, prolongam-se por horas ou são acompanhadas por vômitos ou sintomas neurológicos focais.
Autor(a):
Felipe Resende Nobrega
Residência Médica em Neurologia (UNIRIO) • Mestre em Neurologia (UNIRIO) • Professor de Clínica Médica da UNESA
Referências bibliográficas:
- Upadhyaya P, Nandyala A, Ailani J. Primary Exercise Headache. Curr Neurol Neurosci Rep. 2020; 20(5): 9. doi: 10.1007/s11910-020-01028-4.
- Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018; 38:1.
- Rabiee B, Mohammadinejad P, Kordi R, Yunesian M. The Epidemiology of Exertional Headache in the General Population of Tehran, Iran. Headache 2015; 55:1225. doi: 10.1111/head.12610
- Halker RB, Vargas BB. Primary exertional headache: updates in the literature. Curr Pain Headache Rep 2013; 17:337. doi: 10.1007/s11916-013-0337-8
Você sabe identificar o que é uma enxaqueca vestibular?

A enxaqueca vestibular é um termo usado para descrever vertigem episódica ou sintomas vestibulares atribuídos à enxaqueca. Embora essa associação tenha sido observada desde 1873, ela permanece uma entidade mal definida e compreendida. Alguns fatores que têm dificultado o estudo desta condição são as manifestações clínicas que variam e se sobrepõem a outros distúrbios vestibulares, a compreensão limitada da fisiopatologia subjacente da enxaqueca e a falta de marcadores biológicos ou testes específicos.
A maioria dos pacientes apresentam cefaleia do tipo enxaqueca e sintomas vestibulares episódicos sem outros sintomas neurológicos. Isso distingue a enxaqueca vestibular da enxaqueca com aura do tronco cerebral, na qual pelo menos dois sintomas da circulação posterior são necessários.
Uma história de enxaqueca geralmente precede o desenvolvimento da enxaqueca vestibular, muitas vezes por vários anos. Os principais sintomas clínicos são:
- Vertigem não provocada– Os pacientes geralmente descrevem episódios de vertigem que podem ser internos (uma falsa sensação de movimento do próprio corpo) ou externos (uma falsa sensação de que o ambiente está girando) e frequentemente agravada por mudanças de posição.
- Enxaqueca– Um ou mais sintomas de enxaqueca (fotofobia, fonofobia e/ou aura visual) frequentemente ocorrem durante os episódios de vertigem. Embora a vertigem possa ocorrer com ou sem cefaleia, as enxaquecas estão presentes em 50 a 94% das crises vestibulares. Dentro de um episódio, a vertigem pode ocorrer durante ou imediatamente antes da cefaleia.
- Sintomas auditivos– A fonofobia ocorre em dois terços dos pacientes. Os sintomas de zumbido bilateral ou unilateral, plenitude e deficiência auditiva subjetiva podem fazer parte do quadro. Desta forma, sua presença não diferencia imediatamente a doença de Meniére da enxaqueca vestibular.
Características da enxaqueca vestibular
Quanto as características principais de um episódio de enxaqueca vestibular, merecem destaque:
- Duração– É variável, onde a vertigem aguda pode durar horas, mas a intolerância ao movimento e a instabilidade podem persistir por um dia ou mais. É raro os sintomas persistirem por mais de 72 horas.
- Frequência– Os episódios podem ocorrer várias vezes ao dia; mais comumente, eles são relatados algumas vezes a cada ano.
- Gatilhos– Os gatilhos (privação de sono, certos alimentos, estresse etc.) parecem desempenhar um papel semelhante na enxaqueca vestibular, assim como em outras formas de enxaqueca. Gatilhos visuais e de movimento também são descritos.
Sem um achado de exame ou teste diagnóstico específico, a enxaqueca vestibular é um diagnóstico clínico.
Os critérios da Classificação Internacional de Distúrbios de Cefaleia (ICHD-3) para enxaqueca vestibular são:
- Uma história atual ou passada de enxaqueca (com ou sem aura)
- Pelo menos cinco episódios atendendo aos dois critérios a seguir:
- Sintomas vestibulares de intensidade moderada ou grave, com duração de 5 minutos a 72 horas, e
- Pelo menos 50% dos episódios associados a pelo menos um dos três critérios de enxaqueca:
- Cefaleia com pelo menos duas das seguintes características (unilateral, pulsante, intensidade moderada ou grave, que piora com a atividade)
- Fotofobia e fonofobia
- Aura visual
- Se os sintomas não são mais bem explicados por outro diagnóstico
Na prática clínica, os pacientes com episódios espontâneos recorrentes de vertigem preenchem mais frequentemente os critérios para enxaqueca vestibular provável do que para enxaqueca vestibular definitiva. Diante de um diagnóstico provável, é importante considerar o tratamento de qualquer outro distúrbio vestibular coexistente ou sobreposto e reavaliar o diagnóstico, se o paciente não responder ao tratamento ou se as características clínicas mudarem.
Referências bibliográficas:
- Lempert T, Brevern, MV. Vestibular Migraine. Neurol Clin. 2019 Nov;37(4): 695-706.
- Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018; 38:1.
- Formeister EJ, Rizk HG, Kohn MA, Sharon JD. The Epidemiology of Vestibular Migraine: A Population-based Survey Study. OtolNeurotol 2018; 39:1037.
- Winnick A, Sadeghpour S, Otero-Millan J, et al. Errors of Upright Perception in Patients With Vestibular Migraine. Front Neurol 2018; 9:892.
- Huang TC, Wang SJ, Kheradmand A. Vestibular migraine: An update on current understanding and future directions. Cephalalgia 2020; 40:107.
Demência de início precoce em adultos: como diagnosticar?

O diagnóstico e o tratamento do comprometimento cognitivo progressivo no adulto jovem requerem uma abordagem diferente daquela dos pacientes mais velhos. Em particular, o diagnóstico diferencial é muito mais amplo e frequentemente pede uma avaliação mais extensa, que inclui a investigação de distúrbios comuns e raros. No entanto, as causas mais comuns de demência de início precoce são as mesmas em adultos jovens e idosos: doença de Alzheimer, demência vascular e demência frontotemporal.
De acordo com o DSM-V, a demência é definida como comprometimento cognitivo adquirido em um ou mais domínios da cognição, que representa um declínio significativo de linha de base anterior e interfere na independência nas atividades diárias. Embora a terminologia varie, demências de origem precoce são casos que ocorrem em adultos entre 18 e 65 anos de idade.
A demência deve ser diferenciada dos sintomas de perda de memória ou mudança de personalidade/comportamento, que não são progressivos ou provocados por doenças sistêmicas subjacentes, medicamentos, distúrbios psiquiátricos ou patologia cerebral estrutural.
Os objetivos da avaliação clínica são:
- Determinar o padrão de déficits cognitivos e comportamentais, seu curso e progressão no tempo e até que ponto eles afetam as funções diárias.
- Determinar o envolvimento do sistema nervoso de forma global, incluindo os sistemas motor, sensitivo e cerebelar.
- Determinar se há envolvimento sistêmico.
Causas possíveis de Demência de início precoce
- Demências Neurodegenerativas: Doença de Alzheimer, Demência frontotemporal, Demência por corpúsculo de Lewy, Demência da Doença de Parkinson, Paralisia Supranuclear progressiva, Degeneração corticobasal e Atrofia de múltiplos sistemas.
- Doenças Vasculares: Demência Vascular, CADASIL, Angiopatia amilóide cerebral e Vasculite.
- Doenças Infecciosas: Doença priônica, HIV, Encefalite herpética, Neurossífilis, Doença de Whipple e Leucoencefalopatia multifocal progressiva.
- Doenças Inflamatórias e autoimunes: Esclerose Múltipla, Neurosarcoidose, encefalopatia paraneoplásica e encefalopatia autoimune.
- Doenças metabólicas: Doença mitocondrial, Leucodistrofias de início da fase adulta e Leucoencefalopatia hereditária.
- Outras Causas: Encefalopatia traumática crônica, Hidrocefalia de pressão normal, Doença de Huntington, Doença de Wilson, entre outras.
Além de uma anamnese e um exame físico e neurológico bem detalhado para a demência de início precoce , uma avaliação complementar mais ampla pode ser necessária. Como já descrito, vários diagnósticos diferenciais de distúrbios comuns e raros podem ser realizados:
- Teste cognitivo – Semelhante ao de um paciente idoso, importante realizar testes neuropsicológicos completos para excluir outras etiologias, como distúrbios psiquiátricos e apresentações funcionais.
- Laboratórios de rotina – Laboratórios como hemograma completo, painel metabólico básico, testes de função hepática, estudos da tireoide, vitamina B12 e exame de urina, sorologias para sífilis e HIV. É preciso excluir doenças sistêmicas potencialmente causadoras.
- Neuroimagem estrutural – A ressonância magnética do crânio é um componente importante da avaliação inicial, tanto para excluir patologias estruturais quanto para procurar “pistas” para a etiologia da demência subjacente, por exemplo:
- O padrão de atrofia cerebral pode ajudar a distinguir entre várias doenças neurodegenerativas.
- A presença de pequenas isquemias corticais ou subcorticais antigas, comumente observadas na demência vascular.
- Lesões de substância branca, que podem ser vistas em doenças desmielinizantes adquiridas, como EM (geralmente assimétrica) ou uma leucoencefalopatia de início na idade adulta ou outro distúrbio neurometabólico (frequentemente simétrico).
- Sinal hiperintenso, em imagens ponderadas em DWI, FLAIR e T2, envolvendo o córtex cerebral e corpo estriado, cabeça caudada e putâmen é o padrão mais comum em ressonância magnética de pacientes com doença de Creutzfeldt-Jakob esporádica. Em particular, o envolvimento do giro frontal superior, lóbulo parietal superior, giro cingulado e córtex insular é comum; o envolvimento límbico isolado é raro; e o córtex perirolândico geralmente é poupado.
- Hidrocefalia, conforme observada na hidrocefalia idiopática e de pressão normal secundária (NPH).
- Exame do Líquor (LCR): A punção lombar é sugerida na maioria dos pacientes após a neuroimagem. A análise do LCR pode ajudar a identificar doenças infecciosas e autoimunes. A doença de Alzheimer de início precoce é apoiada por um perfil de peptídeo â amilóide baixo e níveis elevados de tau e fosfo-tau. Laboratórios adicionais são considerados em cada caso, a depender da suspeita diagnóstica, como por exemplo pesquisa proteína 14-3-3 na doença de Creutzfeldt-Jakob.
- Eletroencefalograma – Em casos selecionados, particularmente quando há suspeita de doença priônica.
- Neuroimagem avançada – Angiotomografia ou angioressonância, tomografia por emissão de pósitron (PET) ou tomografia por emissão de fóton único (SPECT).
- Teste genético – Pode ser útil para estabelecer a etiologia em pacientes selecionados. O teste em si ainda é relativamente caro. Uma história familiar clara deve ser documentada em casos suspeitos, com a ressalva de que uma história familiar pode nem sempre ser clara ou disponível. Dentre as demências neurodegenerativas, o teste genético é mais solicitado para CADASIL, Doença de Huntington, formas autossômicas dominantes de demência de Alzheimer e pacientes com demência frontotemporal, particularmente quando há doença do neurônio motor associada. O teste genético também é útil nas leucodistrofias de início na idade adulta e outras doenças neurometabólicas.
Referências bibliográficas:
- Mendez, M. F.Early-onset Alzheimer Disease and Its Variants. CONTINUUM: Lifelong Learning in Neurology, 2019. 25(1), 34–51.
- Cations M, Withall A, Low LF, Draper B. What is the role of modifiable environmental and lifestyle risk factors in young onset dementia? Eur J Epidemiol 2016; 31:107.
- Jang YK, Kwon H, Kim YJ, et al. Early- vs late-onset subcortical vascular cognitive impairment. Neurology 2016; 86:527.
- Rossor MN, Fox NC, Mummery CJ, et al. The diagnosis of young-onset dementia. Lancet Neurol 2010; 9:793.
COVID-19 May Damage Blood Vessels in the Brain

Until now, the neurological manifestations of COVID-19 have been believed to be a result of direct damage to nerve cells. However, a new study suggests that the virus might actually damage the brain’s small blood vessels rather than nerve cells themselves.
A postmortem analysis found abnormalities in the brains of a small sample of patients with COVID-19, suggesting inflammation and vascular damage to the brain stem and olfactory bulb. The findings add further weight to previous research into neurological complications from COVID-19, according to Anna Cervantes, MD. Cervantes is assistant professor of neurology at the Boston University and has been studying the neurological effects of COVID-19, though she was not involved in this study. “I can tell from my personal experience, and things we’ve published on and the literature that’s out there – there are patients that are having complications like stroke that aren’t even critically ill from COVID. We’re seeing that not in just the acute setting, but also in a delayed fashion. Even though most of the coagulopathy is largely venous and probably microvascular, this does affect the brain through a myriad of ways,” Cervantes said.
The research was published online Jan. 12 in the New England Journal of Medicine. Myoung Hwa Lee, PhD, was the lead author.
The study included high resolution magnetic resonance imaging and histopathological examination of 13 individuals with a median age of 50 years. Among 10 patients with brain alterations, the researchers conducted further studies in 5 individuals using multiplex fluorescence imaging and chromogenic immunostaining in all 10.
The team conducted conventional histopathology on the brains of 18 individuals. Fourteen had a history of chronic illness, including diabetes, and hypertension, and 11 had died unexpectedly or been found dead. Magnetic resonance microscopy revealed punctuate hypo-intensities in nine subjects, indicating microvascular injury and fibrinogen leakage. Histopathology using fluorescence imaging showed the same features. Collagen IV immunostaining showed thinning of the basal lamina of the endothelial cells in five patients. Ten patients had congested blood vessels and surrounding fibrinogen leakage, but comparatively intact vasculature. The researchers interpreted linear hypo-intensities as micro-hemorrhages.
The researchers found little perivascular inflammation, and no vascular occlusion. Thirteen subjects had perivascular-activated microglia, macrophage infiltrates, and hypertrophic astrocytes. Eight had CD3+ and CD8+ T cells in the perivascular spaces and in lumens next to endothelial cells, which could help explain vascular injury.
The researchers found no evidence of the SARS-CoV-2 virus itself, despite efforts using polymerase chain reaction with multiple primer sets, RNA sequencing within the brain, or RNA in situ hybridization and immunostaining. Subjects may have cleared the virus by the time they died, or viral copy numbers could have been below the detection limit of the assays.https://0dc17f7ef7d7ab7b99f73bdd35c62cde.safeframe.googlesyndication.com/safeframe/1-0-37/html/container.html
The researchers also obtained a convenience sample of subjects who had died from COVID-19. Magnetic resonance microscopy, histopathology, and immunohistochemical analysis of sections revealed microvascular injury in the brain and olfactory bulb, despite no evidence of viral infection. The authors stressed that they could not draw conclusions about the neurological features of COVID-19 because of a lack of clinical information.
Cervantes noted that limitation: “We’re seeing a lot of patients with encephalopathy or alterations in their mental status. A lot of things can cause that, and some are common in patients who are critically ill, like medications and metabolic derangement.”
Still, the findings could help to inform future medical management. “There’s going to be a large number of patients who don’t have really bad pulmonary disease but still may have encephalopathy. So if there is small vessel involvement because of inflammation that we might not necessarily catch in a lumbar puncture or routine imaging, there’s still somebody we can make better (using) steroids. Having more information on what’s happening on a pathophysiologic level and on pathology is really helpful.”
The study was supported by internal funds from the National Institute of Neurological Disorders and Stroke. Cervantes has no relevant financial disclosures.
This article originally appeared on MDedge.com, part of the Medscape Professional Network.
Medscape Medical News © 2021 WebMD, LLC
Cite this: COVID-19 May Damage Blood Vessels in the Brain – Medscape – Jan 21, 2021.