Angiologia
L’hypertension pulmonaire – Partie II : prise en charge

Étienne-Marie JUTANT, service de pneumologie – Soins intensifs, Hôpital Bicêtre, Le Kremlin-Bicêtre
La prise en charge de l’hypertension pulmonaire (HTP) est spécifique à chaque groupe d’HTP et doit être réalisée en centre expert. En France, la prise en charge est organisée par le réseau national de l’HTP structuré en un Centre de référence de l’HTP (Hôpital Bicêtre, AP-HP) et en 25 Centres de compétences du réseau national (http://www.reseau-htap.fr).
Prise en charge thérapeutique de l’HTP du groupe 1 (HTAP)
La prise en charge repose sur des traitements généraux et des traitements de l’HTAP. Lorsque la maladie évolue, la transplantation pulmonaire reste le seul traitement possible et les patients doivent être évalués précocement en centre de transplantation pulmonaire.
Traitements spécifiques
Diurétiques
Ils permettent de réduire la volémie et d’améliorer les symptômes, en association avec un régime hyposodé. Le furosémide et la spironolactone sont les plus prescrits. Leur posologie doit être régulièrement réévaluée.
Anticoagulants oraux
Les anti-vitamine K (AVK) sont recommandées chez les patients atteints d’HTAP idiopathique, héritable ou induite par la prise d’anorexigènes avec un objectif d’INR entre 1,5 et 2,5. Cette recommandation est de faible grade et le traitement doit être envisagé avant tout en prenant en compte la balance bénéfice/risque pour chaque patient.
Oxygénothérapie
L’oxygénothérapie de longue durée est recommandée en cas d’hypoxémie sévère (PaO2 < 60 mmHg).
Mesures générales
Activité physique adaptée
L’exercice physique intense chez les patients at eints d’HTAP expose au risque de mort subite, mais un mode de vie sédentaire peut participer à la limitation fonctionnelle à l’effort. Un compromis est donc nécessaire avec des efforts guidés par les symptômes.
Vaccination
La vaccination antigrippale et pneumococcique est recommandée.
Grossesse et contraception
La grossesse est contre-indiquée, car à risque de décès pour la mère et de l’enfant avec une mortalité maternelle d’environ 30 %. De plus, les antagonistes des récepteurs de l’endothéline sont tératogènes. C’est pourquoi une contraception est indispensable pour les femmes en âge de procréer. Certains médicaments de l’HTAP (comme le bosentan) inhibent l’activité des estroprogestatifs oraux, c’est pourquoi la contraception généralement prescrite est une contraception par dispositif intra-utérin ou par progestatifs seuls. Si une grossesse survient, elle doit être prise en charge dans un centre expert où une interruption médicale de grossesse sera proposée. Si la grossesse est poursuivie, l’HTAP doit être traitée par des traitements spécifiques non tératogènes et l’accouchement programmé en centre expert en collaborat ion avec une équipe anesthésique, obstétricale et pédiatrique.
Chirurgie et anesthésie
L’anesthésie générale est à risque en raison des modifications hémodynamiques entraînées par l’anesthésie et la chirurgie. C’est pourquoi le rapport bénéficerisque de toute chirurgie doit être évalué de façon multidisciplinaire et l’intervention planifiée et réalisée après un bon contrôle de la maladie.
Médicaments contre-indiqués
Les bêtabloquants baissent le débit cardiaque et doivent être arrêtés lorsque cela est possible.
Médicaments de l’HTAP
Les inhibiteurs calciques
Ils sont réservés aux patients ayant une HTAP idiopathique, héritable ou induite par les médicaments et toxiques et présentant une réponse positive au test de vasodilatation au NO réalisé lors du cathétérisme cardiaque droit (moins de 10 % des HTAP). Ils sont contre-indiqués en l’absence de réponse au test au NO. Les effets secondaires principaux sont la survenue d’hypotension artérielle et d’oedèmes des membres inférieurs. L’efficacité du traitement doit être suivie par une évaluation hémodynamique 3 à 4 mois après le début du traitement. En cas de ré ponse thérapeutique, ce traitement doit être poursuivi au long cours. En cas d’échec thérapeutique, il faut alors l’arrêter et envisager les autres traitements de l’HTAP.
Traitements spécifiques de l’HTAP
La recherche des 25 dernières années sur les mécanismes de l’HTAP a permis le développement de 14 médicaments de l’HTAP, ciblant trois voies principales de la dysfonction endothéliale pulmonaire : la voie de la prostacycline, la voie de l’endothéline, la voie du NO/GMPc (figure 1). Ils sont vasodilatateurs et ont des propriétés anti-remodelage.

Figure 1. Classes thérapeutiques dans l’HTAP ciblant les 3 voies de la dysfonction endothéliale.
GMPc : guanosine monophosphate cyclique ; NO : monoxyde d’azote
La voie de la prostacycline est principalement représentée par L’époprosténol et le tréprostinil. L’époprosténol est le médicament le plus efficace, mais du fait de sa courte demi-vie, son administration est réalisée par voie intraveineuse continue, par l’intermédiaire d’un cathéter veineux central sous-clavier tunnelisé relié à une pompe. Son utilisation nécessite une éducation du patient en milieu hospitalier. Il a montré son efficacité en termes d’amélioration de la capacité à l’exercice, de paramètres hémodynamiques et de survie. Les effets secondaires sont fréquents et sont liés au mode d’administration (infections de cathéter, dysfonctions de pompe…) ou à l’imprégnation du traitement (douleurs de mâchoires, troubles digestifs, bouffées de chaleur, céphalées, érythème…). Le traitement ne doit jamais être interrompu de façon brutale, car cela peut entraîner une défaillance cardiaque droite sévère. Le tréprostinil a une demi-vie plus longue que l’époprosténol et est administré par voie sous-cutanée par une mini-pompe semblable à celles utilisées pour l’insuline. Les douleurs et l’inflammation au site d’injection sont le principal obstacle à leur utilisation en pratique courante. Le sélexipag est un agoniste sélectif des récepteurs à la prostacycline, administré par voie orale, mais son efficacité est moindre que les formes injectables de prostacycline et les effets secondaires (céphalées, douleurs, troubles digestifs) nécessitent une titration très progressive avec suivi rapproché.
Les antagonistes des récepteurs de l’endothéline inhibent les effets de l’endothéline-1 sur la vasoconstriction et la prolifération des cellules musculaires lisses. Le bosentan est un traitement oral qui a montré son efficacité sur la classe fonctionnelle NYHA et l’hémodynamique. Ses effets indésirables sont des céphalées et bouffées de chaleur, mais aussi une élévation des transaminases né cessitant une surveillance mensuelle du bilan hépatique, ain si qu’une possible rétention hydro-sodée. Ce traitement présente aussi des interactions médi camenteuses. L’ambrisentan entraîne moins de cytolyse hépatique et a moins d’interaction médicamenteuse, mais il entraîne une rétention hydrosodée plus importante.
Les médicaments interagissant avec la voie du NO/GMP cyclique sont les inhibiteurs de la phosphodiestérase de type 5 (IPDE5) et les activateurs de la guanylate cyclase soluble. Parmi les IPDE5, le sildénafil et le tadalafil sont les deux traitements approuvés. Leurs effets indésirables sont les céphalées, les bouffées de chaleur et la dyspepsie. Les activateurs de la guanylate cyclase soluble sont représentés par le riociguat qui est approuvé pour l’HTP thromboembolique chronique non accessible à un geste chirurgical. Il nécessite une titration progressive en raison des risques d’hypotension artérielle. L’association du riociguat aux IPDE5 est contre-indiquée du fait du risque d’hypotension artérielle.
De nombreuses autres voies de signalisation impliquées dans le remodelage artériel pulmonaire sont à l’étude dans l’espoir de pouvoir développer de nouveaux médicaments. Un essai de phase 3 est en cours avec le sotatercept, un piège à ligands du transforming growth factor β(TGF β).

Stratégie thérapeutique
Après confirmation du diagnostic d’HTAP dans un centre référent, les mesures générales et les traitements généraux doivent être prescrits en premiers.
Le test de vasoréactivité au NO doit ensuite être réalisé chez les patients ayant une HTAP idiopathique, héritable ou associée aux médicaments et les patients ayant une réponse positive sont traités par des antagonistes calciques à forte dose avec réévaluation à 3 mois.
Pour les non-répondeurs au NO, la stratégie thérapeutique re pose sur l’évaluation du risque de mortalité à 1 an en fonction de paramètres cliniques, biologiques et hémodynamiques (tableau 1). Les patients avec risque faible ou inter médiaire de mortalité sont traités avec les traitements spécifiques de l’HTAP en bithérapie d’emblée associant un IPDE5 et un antagoniste des récepteurs de l’endothéline, sauf chez certains patients pour qui on débutera par une monothérapie (patients âgés avec comorbidités cardiovasculaires, HTAP très légère, hypertension portopulmonaire, HTAP associée au VIH, à une cardiopathie congénitale non traitée). Les patients à risque élevé sont traités d’emblée par une thérapie combinée incluant une prostacycline intraveineuse.
BNP : Brain natriuretic peptide ; IC : index cardiaque ; NT-proBNP : N-terminal pro Brain natriuretic peptide ; NYHA : New York Heart Association ; POD : pression auriculaire droite ; SVO2 : saturation veineuse en O2 ; VE : ventilation ; VCO2 : rejet de dioxyde de carbone ; VO2 : consommation d’oxygène
L’objectif est de maintenir le patient dans un faible risque de mortalité et de proposer une majoration thérapeutique si ces objectifs ne sont pas atteints, après réévaluation du risque de mortalité après 3-6 mois de traitement.
Dans tous les cas, il faut évaluer l’éligibilité à la transplantation pulmonaire en cas de traitement maximal nécessaire d’emblée ou de réponse inadéquate à une thérapie combinée initiale. La stratégie thérapeutique est présentée dans la figure 2.

Figure 2. Stratégie thérapeutique dans l’HTAP issue du 6e Congrès mondial de l’HTAP.

Prise en charge des HTP du groupe 2
Le traitement des HTP du groupe 2 repose sur le traitement de la cardiopathie gauche associée et le traitement des comorbidités associées. Il n’existe pas de preuve de l’efficacité des thérapeutiques de l’HTAP dans les HTP de groupe 2 et celles-ci ne doivent donc pas être utilisées.
Prise en charge des HTP du groupe 3
Il n’y a pas de thérapie spécifique pour l ’HTP de groupe 3. Les patients hypoxiques doivent recevoir une oxygénothérapie et la maladie respiratoire sous-jacente doit être traitée. Il n’y a pas de preuve de l’efficacité des thérapeutiques de l -’HTAP et celles-ci ne doivent pas être utilisées. Certains patients avec HTP sévère paraissant « disproportionnée » à l’atteinte pulmonaire doivent être adressés à un centre de référence ou de compétences de l’HTP pour discuter de la meilleure stratégie thérapeutique.
Prise en charge des HTP du groupe 4
Le traitement de référence est l’endartériectomie pulmonaire, c’est-à-dire le retrait chirurgical du matériel organisé qui obstrue les artères pulmonaires. L’indication chirurgicale repose sur la local isat ion de l ’obstruction (proximale ou distale), la corrélation entre les données hémodynamiques et le degré d’obstruction, les comorbidités. Les patients inopérables peuvent à présent bénéficier soit de traitements médicaux soit de désobstruction par angioplastie pulmonaire et ces traitements peuvent être combinés. Tous les patients doivent être impérativement évalués en centres experts.
Prise en charge des HTP du groupe 5 (mécanisme non clair et/ou multifactoriel)
Le traitement de la maladie causale est la base du traitement. Il n’existe pas d’essais contrôlés sur l’usage des thérapeutiques de l’HTAP dans ce groupe.
Conclusion
- La prise en charge de l’HTP est différente pour chaque groupe d’HTP.
- Plusieurs médicaments ont été développés pour l’HTAP en 25 ans pour viser la dysfonction endothéliale et la recherche se poursuit pour viser le remodelage artériolaire irréversible à l’origine de la maladie.
Publié dans OPA Pratique
Références
Cliquez sur les références et accédez aux Abstracts sur 
Galiè N et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Socie Rechercher l’abstract
Galiè N et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J 2019. Rechercher l’abstract
Hoeper MM et al. Intensive care, right ventricular support and lung transplantation in patients with pulmonary hypertension. Eur Respir J 2019. Rechercher l’abstract
Uno de cada cuatro pacientes con covid-19 en UCI sufre trombosis
Valle de Hebrón y Germans Trias publican la mayor serie mundial hasta ahora sobre esta complicación, que cursa asintomática en un 62% de los casos atendidos en intensivos.
Un estudio de los Hospitales Universitarios Valle de Hebrón de Barcelona y Germans Trias de Badalona, llevado a cabo en abril de 2020, concluye que uno de cada cuatro pacientes graves con con-19 que ingresan en la UCI sufren trombosis venosa o pulmonar y que esta es asintomática en un 62% de ellos. Los resultados han sido publicados en European Journal of Vascular and Endovascular Surgery, la revista científica más prestigiosa en cirugía vascular.
Sergi Bellmunt, jefe del Servicio de Angiología, Cirugía Vascular y Endovascular del Hospital Valle de Hebrón e investigador del Valle de Hebrón Instituto de Investigación (VHIR), ha explicado a Diario Médico que los dos hospitales, del Instituto Catalán de la Salud (ICS), aprovecharon la situación excepcional de tantos pacientes ingresados a la vez por covid-19 en UCI para realizar un estudio que por ahora recoge la mayor muestra al respecto en la bibliografía mundial: 230 casos. Destaca Bellmunt que se sometieron a una revisión de siete meses antes de su publicación. El estudio se hizo en dos días con pacientes ingresados en los dos centros en ese momento pero hay seguimiento a 7 y 30 días y, pronto, a seis meses.
Según ha informado hoy el Valle de Hebrón, el trabajo nació en la primera ola de la pandemia, cuando los profesionales de los hospitales comenzaron a recibir muchos pacientes graves con covid-19 con sospecha de algún tipo de trombosis. Con el objetivo de conocer cuál era el riesgo de sufrir este tipo de complicaciones y establecer qué seguimiento necesitan estos pacientes, los Servicios de Angiología y Cirugía Vascular de los dos hospitales pusieron en marcha el estudio en colaboración con los servicios de Medicina Intensiva y de Anestesiología, Reanimación y Tratamiento del Dolor de Valle de Hebrón y el Servicio de Medicina Interna del Germans Trias.
Los investigadores realizaron una ecografía a cada uno de los 230 pacientes ingresados en las UCI, en un intervalo de tiempo de 48-72h en total. Estas pruebas se hicieron en poco tiempo para minimizar la exposición de los profesionales sanitarios a la covid-19 en un momento complicado de la pandemia respecto a casos y disponibilidad de equipos de protección individual. El objetivo fue determinar si estos pacientes tenían complicaciones tromboembólicas como trombosis venosa profunda o embolia pulmonar.
En el momento de la ecografía, se detectaron 58 pacientes (un 25,2%) que tenían trombosis venosas y/o embolias pulmonares. De estos, solo un 32,8% (lo cual supone un 7% del total de pacientes) eran sintomáticos. Los pacientes se siguieron durante una semana para comprobar su evolución y ver si se desarrollaban nuevas complicaciones. En este seguimiento no se realizaron ecografías adicionales a los casos asintomáticos, sino que se basaba en la aparición de eventos con síntomas o de hallazgos casuales. Así, al cabo de siete días se habían detectado nuevos casos de trombosis hasta llegar a 61 pacientes (un 26,5%). De estos, un 37,5% (23 pacientes, un 8,3% del total) tenían síntomas. En concreto, 38 eran asintomáticos, 7 tenían trombosis venosa sintomática en las piernas, 8 tenían embolia pulmonar sintomática y 8 tuvieron tanto trombosis venosa como embolia pulmonar con síntomas.
Causa de estancia más larga
El estudio también muestra que los pacientes con tromboembolismos venosos en las piernas tenían una estancia más larga en la UCI (22 días de media en los casos con trombos y 17 días en los casos sin trombos), aunque no había diferencias en la mortalidad.
“Hemos comprobado que los pacientes con covid-19 grave tienen un riesgo incrementado de tromboembolismo venoso. Eso es debido a la propia infección por SARS-CoV-2 y también a causa de la inmovilización de los pacientes y el tratamiento que necesitan, como la implantación de catéteres”, explica Bellmunt. En relación al alto porcentaje de asintomáticos, indica que “puede ser a causa de que son pacientes que en muchas ocasiones están intubados y no pueden expresar bien si notan algún síntoma. Además, el hecho de estar tumbado en la cama evita que se produzca edema, es decir, que se hinche la pierna, un síntoma muy característico de la trombosis venosa y sin el cual puede ser que pase desapercibida”.
El hecho de que en muchas ocasiones la trombosis no se manifieste como sintomática es importante por el riesgo de complicaciones que comporta. Por ejemplo, un trombo que esté en las venas de la pierna, si no se trata, podría viajar hacia el pulmón y allí producir una embolia pulmonar, que es mucho más grave. Conocer este riesgo puede ayudar a prevenir su aparición, recuerda el Valle de Hebrón.
En base a los resultados del estudio, los autores consideran necesario administrar dosis más elevadas de tratamiento anticoagulante, como la heparina, como profilaxis en los pacientes con covid-19 grave en la UCI. “Sabiendo que tienen un riesgo elevado de trombosis, y teniendo en cuenta que hemos observado pocos casos con hemorragia, sería necesario administrar heparina en dosis más elevadas de lo habitual en estos pacientes, ya desde el momento del ingreso, para evitar que haya complicaciones graves más adelante”, destaca Secundino Llagostera, jefe del Servicio de Angiología y Cirugía Vascular del Hospital Universitario Germans Trias i Pujol. Actualmente, existen ensayos clínicos en marcha, donde participan los hospitales Valle de Hebrón y Germans Trias, que permitirán valorar la dosis de heparina más adecuada en estos pacientes, para equilibrar el riesgo de trombosis y el de hemorragia.
Bellmunt ha precisado a este diario acerca de la terapia profiláctica que no se puede extrapolar pacientes ingresados en UCI a ingresados en planta y a recluidos ambulatoriamente y que los primeros presentan más factores de riesgo de trombosis porque pueden llegar a estar tres semanas encamados, el 70% están intubados y todos llevan catéteres en venas. A su juicio, aumentar la dosis de terapia profiláctica les expone a mayor riesgo de sangrado, aunque han visto pocos casos de este tipo. Dice sobre estudios específicos en curso relativos a dosis totales y profilácticas que alguno ya se ha cerrado porque se ha visto que, ciertamente, los pacientes sangran mucho. La propuesta, manifiesta, es lo que ya están haciendo en su UCI y en las de otros hospitales para poder aumentar dosis: estratificar a los pacientes, porque no todos son iguales.
Y recomienda para los pacientes con covid-19 en la comunidad (no ingresados en hospital) que se tenga en cuenta el riesgo de trombosis en el reposo y otros factores.
Niveles de D-dímero en sangre
Po otro lado, el Valle de Hebrón también destaca que el trabajo estudió los niveles de D-dímero en sangre en los pacientes, un marcador que se relaciona con la presencia de trombosis. Los resultados mostraron que unos niveles más altos de 1500 ng/mL de D-dímero permitirían distinguir aquellos pacientes con tromboembolismo venoso y con más riesgo de desarrollar complicaciones. “Este análisis requiere prudencia a la hora de interpretarlo, ya que el hecho de que esté aumentado no significa necesariamente que haya una trombosis, sino que tiene que ir acompañado de una sospecha clínica”, asevera el centro.
La principal limitación del estudio que explican los autores es el hecho de que se llevó a cabo en un momento crítico de la pandemia que dificultó hacer un seguimiento en profundidad de todos los pacientes durante su estancia en el hospital.
HTA de la grossesse : les complications cardiovasculaires maternelles

Daniel ROTTEN, Gynécologue-obstétricien des hôpitaux, Paris
L’existence d’un lien entre pathologie hypertensive au cours de la grossesse et risque accru de développer des complications cardiovasculaires dans le futur a été mise en évidence par de nombreux auteurs(2). Un antécédent de prééclampsie ou d’hypertension gravidique peut doubler le risque ultérieur de présenter une cardiopathie ischémique ou un accident vasculaire cérébral(3,4). Néanmoins, cette augmentation n’est pas retrouvée dans toutes les publications, du moins avec une telle intensité(5).
Si la corrélation statistique est constituée, le lien physiopathologique entre les deux situations n’est pas établi. Question : la survenue d’une pathologie hypertensive au cours d’une grossesse est-elle simplement le marqueur d’un terrain vasculaire sous-jacent anormal que la grossesse révélerait ? Inversement, est-ce la pathologie hypertensive de la grossesse qui dégrade le système vasculaire ? Dans cette hypothèse, la pathologie hypertensive serait à l’origine d’une atteinte endothéliale ou d’une lésion d’autres structures vasculaires pendant la grossesse, et la lésion persisterait après l’accouchement. La question n’est pas seulement théorique. Dans la seconde hypothèse en effet, la durée pendant laquelle la patiente est soumise à la pathologie hypertensive serait un facteur d’aggravation du pronostic cardiovasculaire ultérieur. Elle devrait donc être raccourcie au maximum.
Une étude de cohorte
Pour essayer d’avancer sur la question, J.I. Rosenbloom et coll. ont exploité un fichier américain, répertoriant de manière exhaustive toutes les hospitalisations survenant dans les établissements de l’état de New York(1). Y figurent en particulier le motif de chaque hospitalisation, codé selon la Classification internationale des maladies, et le code des actes réalisés. Le but primaire de ce fichier est de permettre une analyse de la qualité des soins et des coûts. Mais en croisant les items, il est possible de relier pour une même patiente trois paramètres : les hospitalisations pour un accouchement compliqué de pathologie hypertensive ; le délai entre le diagnostic d’hypertension de la grossesse et la naissance ; enfin, l’existence d’une hospitalisation à distance de la mère pour pathologie cardio- ou cérébro-vasculaire. Le protocole de l’étude est résumé dans l’encadré.

Résultats
Dans l’intervalle de temps de l’étude, le nombre de patientes enceintes hospitalisées pour pathologie hypertensive dont le dossier est analysable est de 22 594. Dans l’immense majorité des cas, le délai entre diagnostic de l’hypertension et accouchement est compris dans l’intervalle 0-7 jours (n = 19 750 ; 87,4 %). Un délai > 7 jours est observé beaucoup plus rarement (n = 2 844 ; 12,6 %). Le délai médian est de 1 jour (durée interquartile : 0-3 jours ; extrêmes : 0-117 jours). Les deux groupes ne s’avèrent pas identiques sur le plan socio-démographique ou des comorbidités. Il y a dans le groupe « expectative prolongée » plus de patientes noires, elles sont plus âgées, présentent moins de prééclampsies sévères, mais plus de prééclampsies surajoutées. Elles présentent également plus de comorbidités. La durée médiane de suivi au décours de l’accouchement est identique dans les deux groupes (5,2 ans).
Principal résultat de l’étude : les événements constitutifs du critère composite (décès et accidents cardiovasculaires et cérébro-vasculaires) sont observés deux fois plus souvent dans le groupe « naissance dans le délai 0-7 jours », en comparaison avec le groupe « expectative prolongée » (tableau 1).
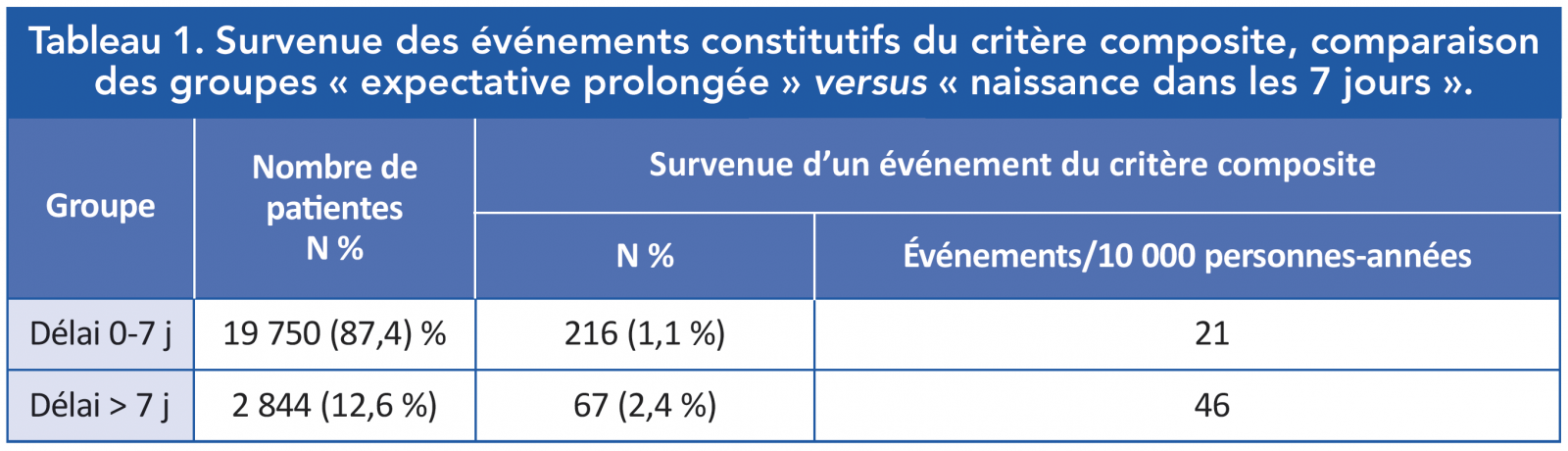
Les événements constitutifs du critère composite le plus souvent observés sont une cardiomyopathie ou une défaillance cardiaque (n = 336). Les autres pathologies cardiovasculaires aiguës ont été rares (n = 14). Il y a eu 88 accidents cérébro-vasculaires et accidents vasculaires cérébraux. Enfin, 32 décès ont été observés (3/10 000 personnes-années).
L’analyse statistique confirme que le hazard ratio ajusté pour les variables socio-démographiques et les comorbidités du groupe « expectative prolongée » est plus élevé que celui mesuré dans le groupe « naissance dans les 7 jours » (tableau 2). Lorsque le délai diagnostic-naissance est analysé comme une variable continue, le HR ajusté est de 1,07 (IC95% : 1,01-1,13) par semaine de grossesse additionnelle. Ces données sont confirmées dans l’analyse par sous-groupes, qu’il s’agisse des seules patientes ayant une prééclampsie sévère, ou des patientes dont l’accouchement a eu lieu dans le délai de 14 jours après le diagnostic (tableau 2).

a. HRa : hazard ratio ajusté pour les variables socio-démographiques et les comorbidités ; b. IC95 % : intervalle de confiance à 95 %.
En résumé, en cas d’HTA de la grossesse (prééclampsie/éclampsie, HTA gravidique, prééclampsie/éclampsie surajoutée à une HTA chronique), la prolongation de la grossesse s’accompagne d’une augmentation de la fréquence ultérieure de survenue de décès et d’accidents cardiovasculaires et cérébro-vasculaires. Cette augmentation est de +7 % par semaine additionnelle de grossesse. On l’observe assez rapidement puisqu’elle apparaît dans les 5 ans qui suivent l’accouchement index . Cette constatation plaide en faveur d’un rôle causal de la pathologie hypertensive dans la survenue ultérieure des complications liées à un dysfonctionnement vasculaire.
Discussion
Dès lors, faut-il modifier nos modalités de prise en charge des HTA de la grossesse ? La stratégie actuelle vise à optimiser le devenir des nouveau-nés. De nombreuses recommandations professionnelles indiquent, chez les patientes ayant une hypertension stabilisée, d’adopter une attitude non interventionniste jusqu’à 34 semaines d’aménorrhée pour les hypertensions sévères, et jusqu’à 37 semaines pour les hypertensions sans élément de sévérité. Mais c’est le pronostic maternel à court terme qui entre dans cette équation. Les données ci-dessus invitent à ne pas être maximaliste en ce qui concerne les bornes de terme en cas de pathologie hypertensive pour tenir compte du pronostic maternel à long terme. Il existe un argument supplémentaire pour ne pas se référer strictement à la règle des 39 semaines d’aménorrhée dans ces cas. Prolonger la grossesse permet d’améliorer le pronostic fœtal et en particulier d’observer une diminution de la mortalité dans la première année de vie. Mais c’est en partie au prix d’une augmentation de la mortinatalité(6). Un éditorial prudent accompagne l’article de J.I. Rosenbloom. Dans ce commentaire, G.N. Smith souligne la possibilité, s’agissant d’une étude rétrospective de cohorte, qu’elle soit entachée de biais(7).
Le premier biais possible porte sur la composition des groupes. Comme not. plus haut, ils ne sont tout à fait comparables ni sur le plan socio-démographique, ni sur celui des comorbidités (les patientes du groupe « expectative prolongée » ont plus de comorbidités). Mais les techniques statistiques utilisées (comparaisons utilisant des HR ajustés et des analyses de propension) ont permis aux auteurs de tenir compte de ces points.
Deuxième biais possible : la détermination de la date de début de la pathologie. Dans l’étude, c’est la date d’hospitalisation qui a été retenue pour la définir. Mais le début clinique réel est dans certains cas antérieur, l’hospitalisation n’ayant pas toujours été immédiate. La constatation d’un délai entre diagnostic de l’hypertension et accouchement compris dans l’intervalle 0-7 jours dans près de 90 % des cas, avec un délai médian de 1 jour, plaide en faveur d’une hospitalisation tardive au cours de l’évolution de la pathologie hypertensive. De plus, la phase infraclinique n’est pas prise en compte.
Au total
En attendant d’autres études de confirmation, les auteurs ne proposent pas de changement radical de conduite. Deux conclusions s’imposent cependant. La première est que, en cas de pathologie hypertensive de la grossesse, il n’est probablement pas indiqué de prolonger la période d’attitude attentiste au-delà du nécessaire. La deuxième est que, dans tous les cas, que la pathologie hypertensive de la grossesse soit causale de la dysfonction endothéliale ou en soit un « simple » marqueur, il y a une augmentation des complications vasculaires ultérieures chez les patientes affect.es. Elles doivent donc bénéficier d’une information, ainsi que d’un suivi approprié à long terme au décours de l’accouchement(4).
Publié dans Gynécologie Pratique
Références
Cliquez sur les références et accédez aux Abstracts sur 
1. Rosenbloom JI et al. Expectant management of hypertensive disorders of pregnancy and future cardio-vascular morbidity. Obstet Gynecol 2020 ; 135 : 27-35. Rechercher l’abstract
2. Simon-Tillaux N et al. Sildenafil for the treatment of preeclampsia, an update. Should we still be enthusiastic? Nephrol Dial Transplant 2019 ; 34 : 1819-26. Rechercher l’abstract
3. Ray JG et al. Cardio-vascular health after maternal placental syndromes (CHAMPS): population-based retrospective cohort study. Lancet 2005 ; 366 : 1797-803. Rechercher l’abstract
4. Graves M et al. Indicateurs du risque cardio-vasculaire liés à la grossesse. Approche des soins de première ligne pour la gestion et la prévention postnatales de maladies futures. Can Family Physician 2019 ; 65 : e505-e512. Rechercher l’abstract
5. Sandvik MK et al. Preeclampsia in healthy women and endothelial dysfunction 10 years later. Am J Obstet Gynecol 2013 ; 209 : 569.e1-10. Rechercher l’abstract
Half of COVID-19 Patients Report Ongoing Fatigue, Study Says

A little more than half of people who recover from COVID-19 report fatigue, even weeks after they get better, according to a new study published in the journal PLOS One.
Regardless of the severity of their infection, about 52% said they still felt extremely tired 10 weeks after contracting COVID-19.
“A lengthy post-infection fatigue burden will impair quality of life and will have significant impact on individuals, employers and healthcare systems,” the researchers from Trinity College Dublin wrote.
The researchers surveyed 128 patients who received COVID-19 care at St. James’s Hospital in Dublin and rated their fatigue scores up to 10 weeks after infection. About 52% — or 67 participants — met the criteria for fatigue, both physically and psychologically. In particular, women and those who had previous diagnoses of depression or anxiety were more likely to report lingering fatigue.
Overall, the patients’ fatigue levels were much higher than the typical scores observed in the general population. However, their scores were lower than the criteria used to indicate chronic fatigue syndrome.
About half of the patients had been hospitalized for COVID-19, and half had received drugs to treat the virus. The researchers didn’t find a link between the persistent fatigue and hospitalization or the severity of the disease.
This lasting fatigue could become a major concern as people recover from COVID-19, the researchers wrote. At the time of the survey, about a third of the participants hadn’t returned to work.
“This is of particular concern, given that it is recommended that post-viral infection return to work should take place after four weeks to prevent deconditioning,” they wrote.
Source:
PLOS One, “Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection.”
WebMD Health News © 2020
Cite this: Half of COVID-19 Patients Report Ongoing Fatigue, Study Says – Medscape – Nov 12, 2020.
Microbleeds Shouldn’t Influence Anticoagulation After Stroke

The presence of microbleeds in the brain should not affect the decision to prescribe anticoagulation to patients after an ischemic stroke, new data suggest.
The new results come from a prespecified substudy of the NAVIGATE-ESUS trial, which compared rivaroxaban to aspirin in patients with embolic stroke of undetermined source (ESUS). This substudy investigated whether patients with microbleeds differ with respect to future risk for intracerebral hemorrhage (ICH) and ischemic stroke if taking rivaroxaban or aspirin.
Dr Ashkan Shoamanesh
“We found that microbleeds increased the risk of ischemic stroke, ICH, and mortality, but this increase was the same regardless of which antithrombotic strategy was used,” lead author Ashkan Shoamanesh, MD, McMaster University, Hamilton, Canada, commented to Medscape Medical News.
“This is the first randomized trial ever to have examined the interaction between microbleeds and anticoagulation vs aspirin on clinical outcomes and, as such, is a valuable contribution to the field,” he added. “Our results suggest that we should not consider microbleeds when making the decision of whether to prescribe an anticoagulant or after an ischemic stroke.”
The main NAVIGATE-ESUS trial was stopped early because of increased bleeding in the rivaroxaban group, and anticoagulation is therefore not indicated for patients with ESUS.
However, Shoamanesh pointed out that the underlying disease mechanisms causing microbleeds are similar across stroke subtypes, so these results are likely generalizable to other types of ischemic stroke, such as cardioembolic strokes due to atrial fibrillation.
“Anticoagulants are very effective in patients with cardioembolic stroke, but we hesitate to use them in patients with microbleeds. These results suggest we should not factor in microbleeds in the decision to use anticoagulation. As microbleeds occur in around 30% of such patients, this will affect clinical practice in a meaningful way,” Shoamanesh said.https://tpc.googlesyndication.com/safeframe/1-0-37/html/container.html
“Our findings also support the continued use of anticoagulation in patients with ischemic stroke who are incidentally found to have these lesions on imaging,” he added.
The study was published online in JAMA Neurology on October 19.
Of the 7213 NAVIGATE-ESUS participants, 3699 (51%) were eligible for the current analysis on the basis of the fact that their baseline clinical MRI reports contained information on microbleeds. Patients with a prior history of symptomatic ICH were excluded from the NAVIGATE-ESUS trial.
Results showed that microbleeds were present in 395 of the 3699 participants (11%). Factors that were independently associated with microbleeds were advancing age (odds ratio [OR] per year, 1.03); East Asian race/ethnicity (OR, 1.57); hypertension (OR, 2.20); multiterritorial infarcts (OR, 1.95); chronic infarcts (OR, 1.78); and occult ICH (OR, 5.23).
The presence of microbleeds was associated with a 1.5-fold increased risk for recurrent stroke, a fourfold increase in risk for ICH, and a twofold increase in risk for all-cause mortality. Strictly lobar microbleeds were associated with an approximately 2.5-fold increased risk for ischemic stroke.
There were no interactions between microbleeds and treatment assignments (rivaroxaban or aspirin) for recurrent stroke, ischemic stroke, or all-cause mortality. The hazard ratios of ICH for patients taking rivaroxaban were similar for patients with microbleeds (HR 3.1) and for those without microbleeds (HR, 3.0).
“In contrast to questions raised from meta-analyses of observational studies, we found no indication of interaction between the effects of rivaroxaban and cerebral microbleeds, including multiple or strictly lobar microbleeds, for the outcome of ICH. Although our analysis lacks the power to confidently exclude such an effect, there were no suggestive numerical trends identified,” the researchers write.
“The underlying cerebral small vessel diseases for which cerebral microbleeds are a marker are prevalent in populations with stroke of all causes; hence, our reported lack of effect modification may be generalizable to other stroke subtypes beyond the ESUS population reported here,” they add.
“Overall, our results and the existing literature from randomized trials and recent meta-analyses do not support the clinical concern regarding antithrombotic therapy in patients with ischemic stroke and cerebral microbleeds,” they conclude.
“We know the presence of microbleeds on MRI increase the risk of ICH during follow-up, which makes us more careful about use of anticoagulation where microbleeds are present. But microbleeds also increase the risk of ischemic stroke,” Shoamanesh commented.https://tpc.googlesyndication.com/safeframe/1-0-37/html/container.html
“While the relative risk of ICH in patients with microbleeds is higher than that for ischemic strokes, because ischemic strokes are much more common, the absolute risk of an ischemic stroke is greater than that of an ICH. And it is absolute risks that are more relevant to clinical practice,” he said.
Shoamanesh stressed that these results should not be extrapolated to patients with a history of ICH. “Whether microbleeds can modify the effect of anticoagulation in ICH survivors towards net harm has yet to be assessed in randomized controlled trials and remains uncertain. MRI substudies of several ongoing trials assessing optimal stroke prevention in ICH survivors with atrial fibrillation, such as the ongoing global ENRICH-AF trial, will ultimately provide more insights in this regard,” he said.
In an accompanying editorial, Laurent Puy, MD, and Charlotte Cordonnier, MD, PhD, Université de Lille, Lille, France, say that Shoamanesh and colleagues have produced more data showing that cerebral microbleeds are markers of the severity of the underlying vessel disease.
“Patients with cerebral microbleeds can be at risk of ICH but, in cohorts of ischemic strokes, they are at even greater risk of recurrent ischemic stroke. In patients at high risk of ischemic stroke, clinicians should stop worrying about a possible bleed and start a solid evidence-based prevention of future ischemic events,” they write.
“The present study could contribute to changing the mindset of clinicians. Because the presence of cerebral microbleeds does not seem to modify the effect of rivaroxaban on clinical outcomes, their use in guiding anticoagulation therapy decision-making is then questionable,” the editorialists conclude. “Current evidence does not justify avoiding antithrombotic medication in patients with stroke based solely on the presence of cerebral microbleeds.”
The study was supported by Bayer AG and Janssen Research and Development LLC. Shoamanesh reports receiving grants, personal fees, or advisory board fees from Bayer AG, Bristol-Myers Squibb, Boehringer Ingelheim, Daiichi-Sankyo, Servier Canada Inc, Janssen, and Bayer Canada during the conduct of the study. Cordonnier reports receiving personal fees from Boehringer Ingelheim and being a steering committee member of the Antithrombotic Treatment With Factor XIa Inhibition to Optimize Management of Acute Thromboembolic Events in Secondary Stroke Prevention (AXIOMATIC-SSP) trial, which is sponsored by Bristol-Myers Squibb.
JAMA Neurol. Published online October 19, 2020. Abstract, Editorial.
Medscape Medical News © 2020
Cite this: Microbleeds Shouldn’t Influence Anticoagulation After Stroke – Medscape – Oct 22, 2020.
#El #microbioma intestinal protege frente a la predisposición genética a la #leucemia
La exposición a estímulos infecciosos podría alterar las bacterias intestinales y estar en el origen de la leucemia en niños susceptibles de padecerla.
El microbioma intestinal, compuesto por el conjunto de genes que forman parte de las bacterias intestinales desde el nacimiento, protege –según un ensayo en modelo animal-, frente a la predisposición genética a desarrollar leucemia. Este hallazgo, que publica Blood, contribuiría al desarrollo de nuevas herramientas para prevenir la enfermedad en los niños con susceptibilidad genética a padecerla.
A pesar de que aún se desconoce el mecanismo molecular específico que la interacción del microbioma en esta patología tumoral, Isidro Sánchez García, del Centro de Investigación del Cáncer de Salamanca y director del ensayo, señala a DM que este ámbito es precisamente una de las prioridades científicas de este grupo de investigación.
El papel de las exposiciones previas
La comprensión del microbioma constituye un campo de investigación en crecimiento. Numerosos estudios sugieren que “la relación entre los microorganismos y el material genético juega un papel relevante en el origen de enfermedades neurológicas o inmunitarias como la leucemia infantil”, aunque “el cambio en el microbioma intestinal vinculado a la susceptibilidad genética se observa tanto en jóvenes como en adultos”, matiza.
El tipo más frecuente de leucemia infantil es la linfoblástica aguda de células B precursoras, causada por una combinación de la susceptibilidad genética del niño al nacer junto a la exposición a ciertas infecciones tras el parto. Las predisposiciones genéticas son frecuentes en los niños y se consideran condición necesaria para el desarrollo de la enfermedad, si bien menos del 1% de estos casos desarrollarán a lo largo de su vida leucemia linfoblástica aguda de células B precursoras.
Es esencial investigar por qué no todos los individuos desarrollan la enfermedad, a pesar de tener predisposición
Según Sánchez García, aunque se conocen determinados factores implicados en el desarrollo de la leucemia infantil, como la exposición a estímulos infecciosos, “resulta fundamental profundizar y describir con precisión por qué, a pesar de tener esta predisposición, no todos los individuos desarrollan la enfermedad”.
Los investigadores han averiguado que, cuando los ratones con predisposición genética son tratados con antibióticos en edades tempranas, se altera su microbioma y este cambio es suficiente para inducir la leucemia, incluso en ausencia de estímulos infecciosos. “En esta situación, los microbios intestinales son distintos a los que tienen los animales no susceptibles a la enfermedad. De hecho, sería posible identificar la predisposición genética de un individuo caracterizando su microbioma.
Los resultados parecen indicar que el desarrollo de leucemia linfoblástica aguda en ratones con predisposición genética está más relacionado con una falta de ‘microbiota comensal’ —la que normalmente contiene el intestino— que con la presencia de bacterias específicas.
La susceptibilidad se relaciona más con una falta de ‘microbiota comensal’ que con la presencia de bacterias específicas
El siguiente paso es llevar a cabo estudios a gran escala dirigidos a determinar si una modificación del microbioma en los niños con predisposición genética a la leucemia linfoblástica aguda de células B puede convertirse en una estrategia exitosa. “Un posible tratamiento podría ser administrar la ‘microbiota comensal’, principalmente lactobacillus, a los ratones susceptibles para poder prevenir el desarrollo de la leucemia, aunque actualmente, se desconoce cuál sería su papel concreto”, concluye Sánchez García.
#IVUS pour #pathologies artérielles périphériques
L’artériographie par soustraction numérique est toujours considérée comme l’examen de référence pour l’évaluation des vaisseaux périphériques, mais ses limites techniques sont bien connues(1,2). Au cours des dernières années, l’échographie intravasculaire (IVUS) a gagné de plus en plus de popularité non seulement comme méthode diagnostique, mais aussi comme outil thérapeutique de guidage des procédures endovasculaires.
Le traitement de la maladie artérielle périphérique symptomatique vise à restorer de manière adéquate le flux sanguin en élargissant le diamètre de la lumière du vaisseau. Dans le traitement de routine, les procédures endovasculaires sont principalement réalisées en utilisant l’artériographie comme méthode de guidage(1-4).
Toutefois, certaines limitations techniques sont bien connues et corrélées à la caractéristique bidimensionnelle des images telles que l’évaluation incorrecte du diamètre du vaisseau, l’analyse incorrecte de la sténose, etc.
Ces limitations sont plus évidentes pour les vaisseaux périphériques dont la morphologie 3D, complexe, doit être analysée(5).
Comme indiqué dans la littérature, l’artériographie fournit des informations sur les caractéristiques de la lumière des artères périphériques, mais sous-estime sévèrement l’étendue de l’athérosclérose pour les patients présentant des pathologies artérielles périphériques (PAD), même pour les vaisseaux « d’apparence normale »(6).
IVUS : un instrument essentiel
Au cours des dernières années, l’IVUS a gagné de plus en plus de popularité dans l’évaluation de la morphologie des vaisseaux périphériques. Parti d’un simple outil de diagnostic, l’IVUS est aujourd’hui devenu un instrument important pour guider les procédures de revascularisation dans le domaine vasculaire périphérique.
Plusieurs avantages sont corrélés avec l’utilisation de l’IVUS. Il permet une visualisation claire de la lumière du vaisseau de l’intérieur avec une évaluation correcte du diamètre luminal, du degré de sténose et de l’extension de la lésion.
En outre, il permet également l’évaluation de la morphologie de la plaque et de sa géométrie. Sur la base des données recueillies, en utilisant l’IVUS, les opérateurs sont en mesure de sélectionner le traitement le plus approprié, tel que l’athérectomie, l’angioplastie simple au ballon ou la pose d’une endoprothèse(7, 8).
Les études
Le premier article portant l’attention sur l’intérêt de l’utilisation de l’IVUS en périphérique a été publié en 1998(9).
Quelques années plus tard, en 2002, C. J. Buckley et al. (10) ont rapporté leur expérience sur 52 patients ; 36 d’entre eux ont reçu la pose d’une endoprothèse iliaque guidée par artériographie et IVUS, tandis que 16 ont été traités conventionnellement en utilisant uniquement l’artériographie. Les auteurs ont souligné combien l’utilisation de l’IVUS augmentait les résultats avec une perméabilité primaire de 100 % après 6 ans. Cette valeur était clairement supérieure aux 69 % rapportés dans les mêmes indications chez les patients chez lesquels l’IVUS n’était pas utilisé. Le motif réside dans le fait que grâce à l’IVUS ils ont été en capacité de mesurer le calibre de l’artère avec plus de précision et donc de choisir le diamètre le plus approprié de stent.

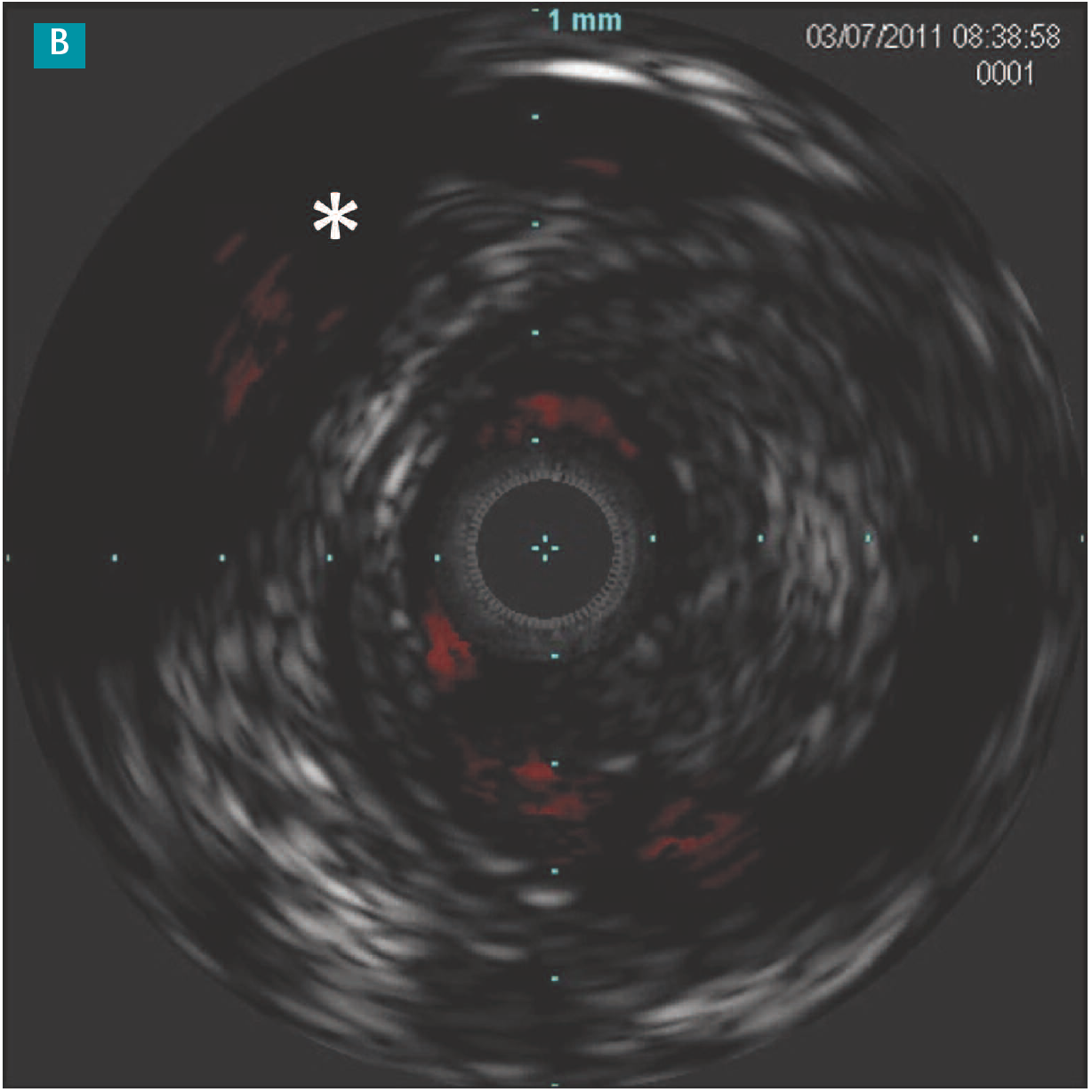
Figure 1 : A : L’artériographie postangioplastie avec ballon actif montre une dissection sans limitation du flux sanguin. B : L’image IVUS montre une dissection intimale large (*). Les vraies et fausses lumières sont clairement identifiables.
L’IVUS a montré qu’après angioplastie, l’élargissement luminal est produit par l’étirement de la paroi artérielle tandis que le volume de la lésion reste relativement constant(11). Il s’agit d’une autre information importante que les opérateurs peuvent utiliser pour sélectionner le dispositif le plus approprié.
Par exemple, en cas de pathologie calcifiée, l’athérectomie est l’outil préférable pour préparer la lésion et l’IVUS peut guider non seulement sur la zone où le plus de plaque est présent, mais aussi lorsque l’abrasion de la plaque peut être considérée comme suffisante(12). Plusieurs articles ont été publiés décrivant les avantages de l’IVUS lors de l’utilisation des dispositifs d’athérectomie(13).
Figure 2 : L’mage IVUS acquise après déploiement du stent dans l’artère fémorale superficielle (AFS) met clairement en évidence la dilatation incorrecte de l’endoprothèse qui n’atteint pas entièrement la paroi artérielle.
L’IVUS joue également un rôle important lors de la recanalisation des occlusions chroniques (CTO). En fait, la position du guide, endoluminal ou sous-intimal, peut facilement être reconnue par ce système. Un succès technique de 97 à 100 % a été décrit avec un temps de réentrée allant de 3 à 10 minutes(14).
En outre, les images d’IVUS transversales des zones d’atterrissage proximale et distale peuvent être une étape cruciale pour la planification de la stratégie et la sélection des dispositifs, comme nous le savons tous par notre exérience sur les occlusions coronaires totales.
La combinaison de l’IVUS et du dispositif de réentrée (Pioneer, Philips Medical) permet, également dans les conditions les plus difficiles, de réintégrer la vraie lumière, ce qui augmente drastiquement le succès technique de la recanalisation endoluminale.
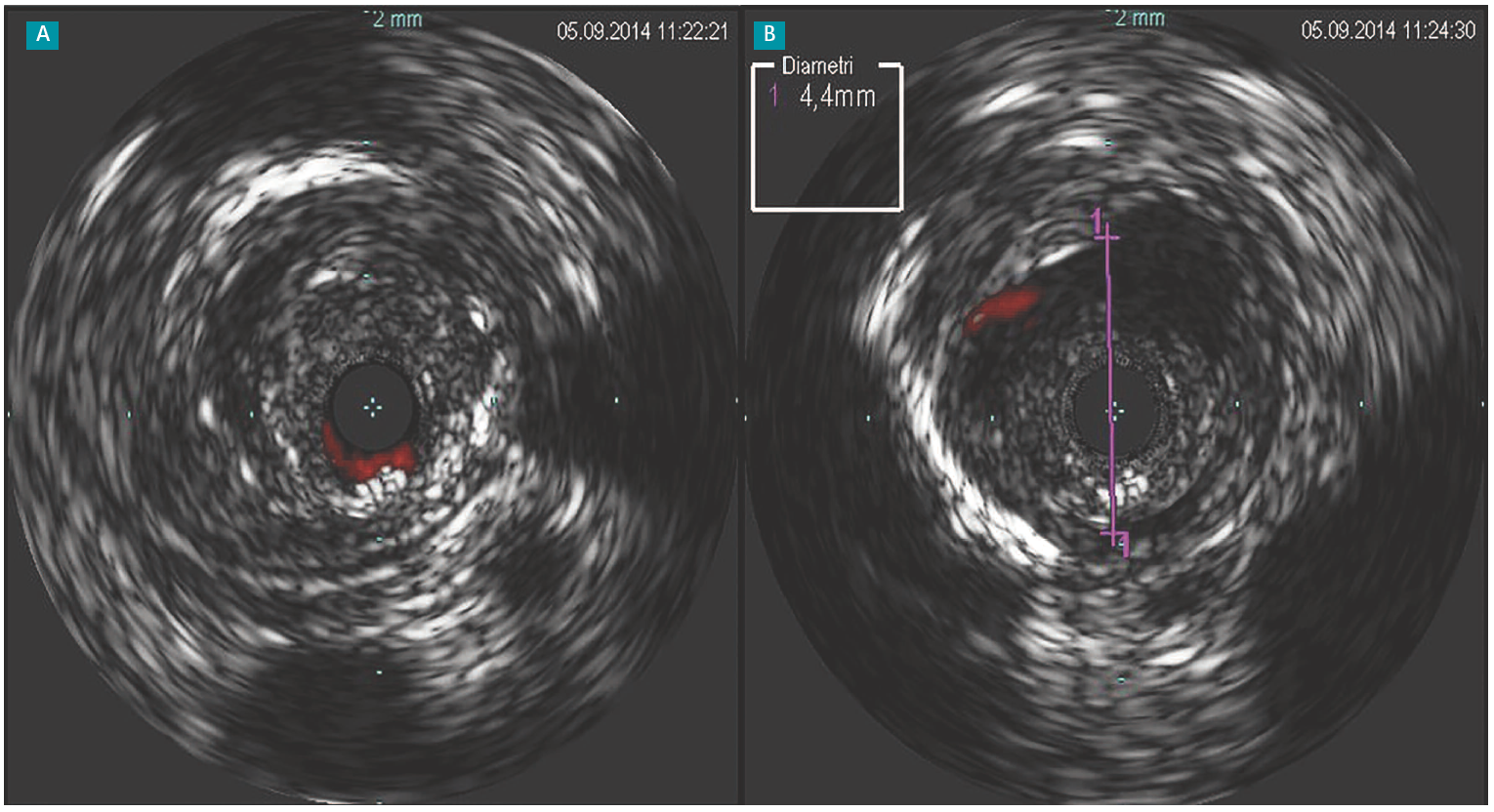
Figure 3 : A : Image IVUS acquise chez un patient avec CTO immédiatement après recanalisation initiale. L’utilisation du CromaFlo (Philips) montre la lumière perméable de faible diamètre autour du cathéter. B : Image après dilatation du segment recanalisé avec un ballonnet de 4 mm. Sur l’image IVUS, le gain de lumière est visible.
D. Kawasaki et al.(14) ont effectué des comparaisons entre la réentrée intraluminale guidée par IVUS et celles non guidées par IVUS et ont démontré une exposition au produit de contraste significativement plus faible en faveur du groupe IVUS (104 ml vs 201 ml ; p < 0,001)(15). En cas de CTO, Y. Takahashi (16) a raporté une nouvelle technique où un cathéter IVUS de 10 MHz a été inséré dans le système veineux pour guider des interventions endovasculaires dans le site artériel. Dans cette étude, l’intervention guidée par IVUS a été proposée à 44 patients avec un taux de succès technique de 96 % dans la recanalisation du vaisseau. Des patients présentant des calcifications modérées ou sévères ont également été inclus dans l’étude. Le taux d’absence de revascularisation de lésion cible (TLR) à 1 an de suivi était de 77,9 %. Il convient de noter que le taux d’endoprothèses était extrêmement faible, ce qui suggère que, dans le cadre d’une CTO, l’approche transveineuse guidée par IVUS peut aider l’opérateur à rester dans la véritable lumière, plutôt que de passer dans l’espace sous-intimal.
Enfin, l’IVUS permettrait également de réduire le volume de produit de contraste et l’exposition aux rayonnements(15,17). Cet avantage semble être très prometteur pour les patients souffrant de diabète, d’insuffisance rénale chronique ou même d’allergies aux produits de contraste. La réduction de l’exposition aux rayonnements est en effet un autre élément important, non seulement pour les patients, mais aussi pour les opérateurs.
Conclusion
- L’IVUS devient de plus en plus populaire grâce aux nombreux avantages rapportés dans la littérature.
- Il peut être considéré non seulement comme un outil d’imagerie d’aide au diagnostic, mais aussi comme un outil thérapeutique de guidage pour les procédures endovasculaires.
- L’IVUS permet une évaluation correcte de la longueur de lésion, du diamètre du vaisseau, de la morphologie de la plaque, de la géométrie de la plaque.
- Il peut augmenter le succès technique dans la recanalisation d’une CTO.
- Le remboursement est le principal facteur limitant son utilisation.
Publié dans L’Interventionnel
Références
Cliquez sur les références et accédez aux Abstracts sur 
1. Adam DJ et al. Bypass versus angioplasty in severe ischemia of the leg (Basil): multicentre, randomised controlled trial. Lancet 2005 ; 366 : 1925-34. Rechercher l’abstract
2. Schillinger Met al. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Engl J Med 2006 ; 354 : 1879-88. Rechercher l’abstract
3. Mustapha JA et al. Percutaneous transluminal angioplasty in patients with infrapopliteal arterial disease: systematic review and meta-analysis. Circ Cardiovasc Interv 2016 ; 9 : e003468. Rechercher l’abstract
4. Popplewell MA et al. Bypass versus angio plasty in severe ischaemia of the leg – 2 (BASIL-2) trial: study protocol for a randomised controlled trial. Trials 2016 ; 17 : 11. doi:10.1186/s13063-015-1114-2 Rechercher l’abstract
5. Kashyap VS et al. angiography underestimates peripheral atherosclerosis: lumenography revisited. J endovasc ther 2008 ; 15 : 117-25. Rechercher l’abstract
6. Arthurs ZM et al. Evaluation of peripheral atherosclerosis: a comparative analysis of angiography and intravascular ultrasound imaging. J Vasc Surg 2010 ; 51 : 933-8 ; discussion 939. Rechercher l’abstract
7. Nakatani S et al. How clinically effective is intravascular ultrasound in interventional cardiology? Present and future perspectives. Expert Rev Med Devices 2013 ; 10 : 735-49. Rechercher l’abstract
8. Nissen SE, Yock P. Intravascular ultrasound. Novel pathophysiological insights and current clinical applications. Circulation 2001 ; 103 : 604-16. Rechercher l’abstract
9. Arko F et al. Use of intravascular ultrasound improves long-term clinical outcome in the endovascular management of atherosclerotic aortoiliac occlusive disease. J Vasc Surg 1998 ; 27(4) : 614-23. Rechercher l’abstract
10. Buckley CJ et al. Intravascular ultrasound scanning improves long-term patency of iliac lesions treated with balloon angioplasty and primary stenting. J Vasc Surg 2002 ; 35 (2) : 316-23. Rechercher l’abstract
11. Jacobs LJ. The evolution of true lumen re-entry. An introduction to the OffRoad™ re-entry catheter system. evtoday.com/articles/2014-mar-supplement/ the-evolution-of-true-lumen-re-entry Rechercher l’abstract
12. Baker AC et al. Technical and early outcomes using ultra-sound-guided reentry for chronic total occlusions. Ann Vasc Surg 2015 ; 29 : 55-62. Rechercher l’abstract
13. Krishnan P et al. Intravascular ultrasound guided directional atherectomy versus directional atherectomy guided by angiography for the treatment of femoropopliteal in-stent restenosis. Ther Adv Cardiovasc Dis 2018 ; 12: 17- 22. Rechercher l’abstract
14. Krishnamurthy VN et al. Intravascular ultrasound-guided true lumen reentry device for recanalization of unilateral chronic total occlusion of iliac arteries: technique and follow-up. Ann Vasc Surg 2010 ; 24 : 487-97. Rechercher l’abstract
15. Kawasaki D et al. Novel use of ultrasound guidance for recanalization of iliac, femoral, and popliteal arteries. Catheter Cardiovasc Interv 2008 ; 71 : 727-33. Rechercher l’abstract
16. Takahashi Y et al. Transvenous intravascular ultrasound-guided endovascular treatment for chronic total occlusion of the infrainguinal arteries. J Endovasc Ther 2017 ; 24 (5) : 718-26. Rechercher l’abstract
17. Mariani J Jr et al. Intravascular ultrasound guidance to minimize the use of io- dine contrast in percutaneous coronary intervention: the MoZart (Minimizing contrast utiliZation with IVUS guidance in coronary angioplasty) randomized controlled trial. Rechercher l’abstract
#Visualizan por primera vez la presencia del #SARS-CoV-2 en la #perniosis o #sabañones de los niños
Se ha demostrado la presencia del coronavirus en células endoteliales mediante biopsia y se ha visualizado en algunas lesiones cutáneas.
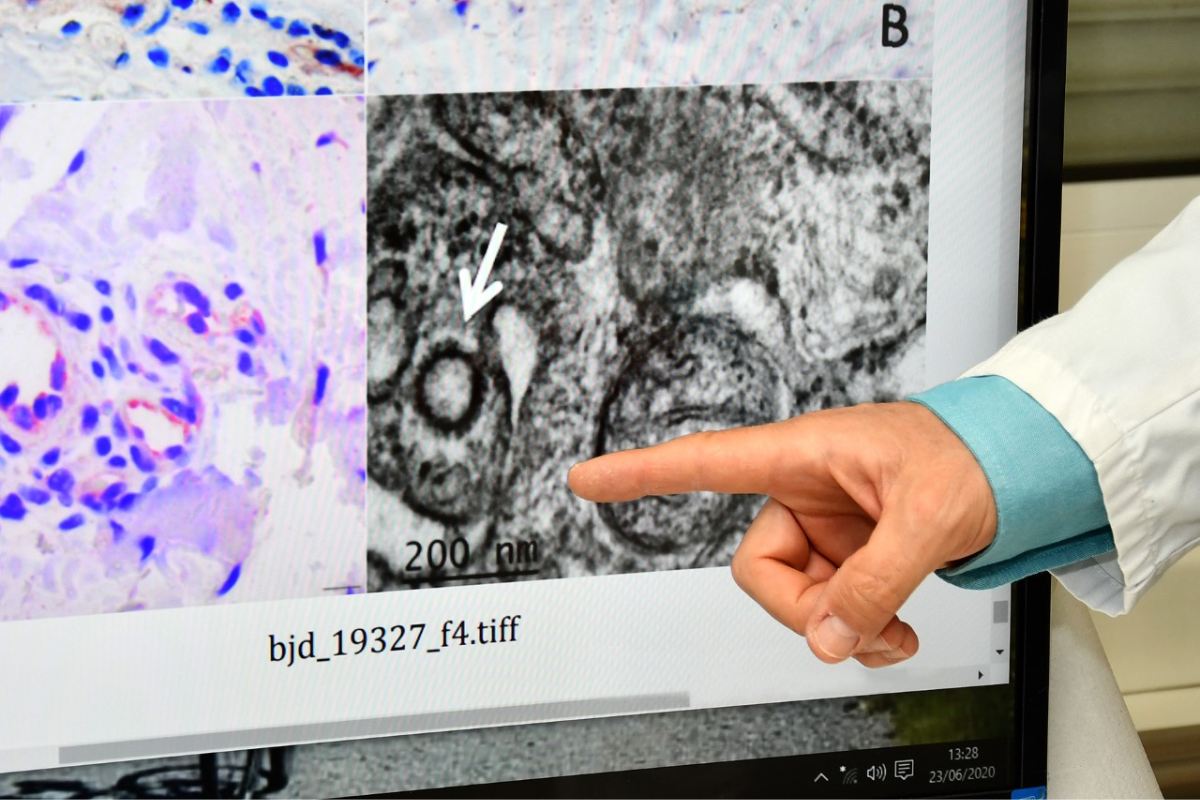
Los equipos de Antonio Torrelo e Isabel Colmenero, de los servicios de Dermatología y Anatomía Patológica, respectivamente, del Hospital Niño Jesús, en colaboración con los servicios de Anatomía Patológica del Hospital 12 de Octubre y de la Fundación Jiménez Díaz, todos de Madrid, han conseguido demostrar “la presencia del coronavirus SAV-CoV-2 en las células endoteliales, visualizándose por primera en el mundo vez el virus en algunas lesiones de la piel que han sido biopsiadas”, explica a DM Torrelo, jefe de Dermatología del Niño Jesús. Paralelamente, el grupo ha descrito la presencia de daño vascular y la formación de pequeños trombos en los vasos afectados, hallazgos coincidentes con los observados y publicados en pacientes adultos afectados por la Covid-19 con enfermedad grave, tanto en la piel como en otros órganos diana.
Los datos de los análisis se publican en el British Journal of Dermatology y en Pediatrics Dermatology y han sido aceptados en otras publicaciones científicas. Torrelo, que es además presidente de la Sociedad Europea de Dermatología Pediátrica prepara, junto con profesionales de países que más cuadros de estas características han reportado, una revisión de todas las manifestaciones Covid en la edad pediátrica.
Con PCR negativa
Según Torrelo, las lesiones se han caracterizado, primero clínicamente y, después, histológicamente. “Lo más llamativo en los niños son las lesiones similares a los sabañones, que son más específicas de Covid y que hasta llegaron a verse 30 en una semana. Las perniosis se han manifestado en niños más cercanos a la adolescencia, que en niños muy pequeños. En neonatos, más esporádicos, hemos visto lesiones de Covid, como púrpura en los pies, por ejemplo. Este tipo de patología, fundamentalmente la perniosis, puede considerarse lesiones Covid, más aún cuando el virus se ha detectado en ellas mediante diversas técnicas de análisis antonomopatológico”.
Las investigaciones han permitido, por tanto, describir las características clínicas de una serie de pacientes con eritema pernio o perniosis, más conocido como sabañones, y otras alteraciones cutáneas relacionadas con la Covid, como lesiones tipo eritema multiforme y lesiones purpúricas en las plantas de los pies.
Curiosamente, señala el dermatólogo, “la mayoría de los pacientes sometidos a estudio habían dado negativo en la PCR y en serología. Probablemente se deba a que el periodo de detección por PCR has pasado o que si la erupción ha sido leve no ha generado anticuerpos, pero el virus sí se encuentra en el endotelio vascular”.
Daño endotelial presente
Estas manifestaciones, según Torrelo, son generalmente una manifestación tardía: el niño que presenta estas manifiestaciones seguramente ha estado expuesto al virus dos o cuatro semanas antes; es como una especie de reacción inmunológica; el virus desaparece de la garganta, pero sigue estando activo en los vasos sanguíneos, al igual que ocurre en los pacientes adultos”.
Colmenero indica que este tipo de lesiones pueden considerarse lesiones Covid y que el daño endotelial a su vez será el responsable, probablemente, de la respuesta inflamatoria que se genera y que incluye vasculitis y trombosis, por ejemplo, que es infrecuente en nuestros pacientes pediátricos. “En niños hablamos más de microangiopatía en pies y manos, que es como un mini-Covid severo, y que en adultos podría explicar la enfermedad más grave”.
Los hallazgos de este grupo de investigadores, son claves para entender la Covid y abren la puerta a futuras investigaciones encaminadas al “diagnóstico diferencial con otras alteraciones cutáneas en niños causadas por otros procesos virales”, según Torrelo, así como buscar los mecanismos que hacen que los niños, en su mayoría, no desarrollen formas graves de la enfermedad.
“Una parte importante de la explicación puede ser que en adulto, el endotelio no es vírgen, como el de los niños, y está sujeto a situaciones que lo han dañado previamente por otras enfermedades de base como la HTA, la diabetes o la obesidad. En niños, por el contrario, se están viendo lesiones en zonas más distales porque son más susceptibles a la isquemia. Pero todo esto son hipótesis que habrá que confirmar”, señala la especialista en anatomopatología.
#La #endotelitis ‘orquesta’ las formas más graves de la #Covid-19
Estudian si el fármaco defibrotide, un protector del endotelio, puede reducir la mortalidad por coronavirus grave.
La evidencia científica está dando la razón a la hipótesis de la implicación del daño vascular, y más concretamente, del endotelio en las formas graves, y mortales, de la Covid-19. Casi a primera vista, el vínculo entre las células endoteliales y la nueva enfermedad es apreciable en los grupos de riesgo: mayores y personas con obesidad, hipertensión y diabetes, todos ellos factores que “se caracterizan por una disfunción vascular preexistente con un metabolismo alterado de las células endoteliales”, apuntaban en Nature Reviews Immunology un grupo de médicos del Laboratorio de Angiogénesis y Metabolismo en el Instituto del Cáncer de Lovaina (Bélgica).
Pero la hipótesis se confirma además en las autopsias y biopsias publicadas, que coinciden en sus hallazgos de endotelitis, rotura de la barrera endotelial y microtrombos en diferentes órganos afectados por el coronavirus. Parece que la endotelitis es una “marca de la casa” del coronavirus SARS-CoV-2, a diferencia de lo que ocurre cuando el distres respiratorio fatal aparece por el virus de la gripe, como apuntaba en The New England Journal of Medicine, un grupo de patólogos encabezado por Maximilian Ackermann, del Helios Hospital Universitario Wuppertal, en Alemania.
#COVID-19 et #maladie thromboembolique veineuse
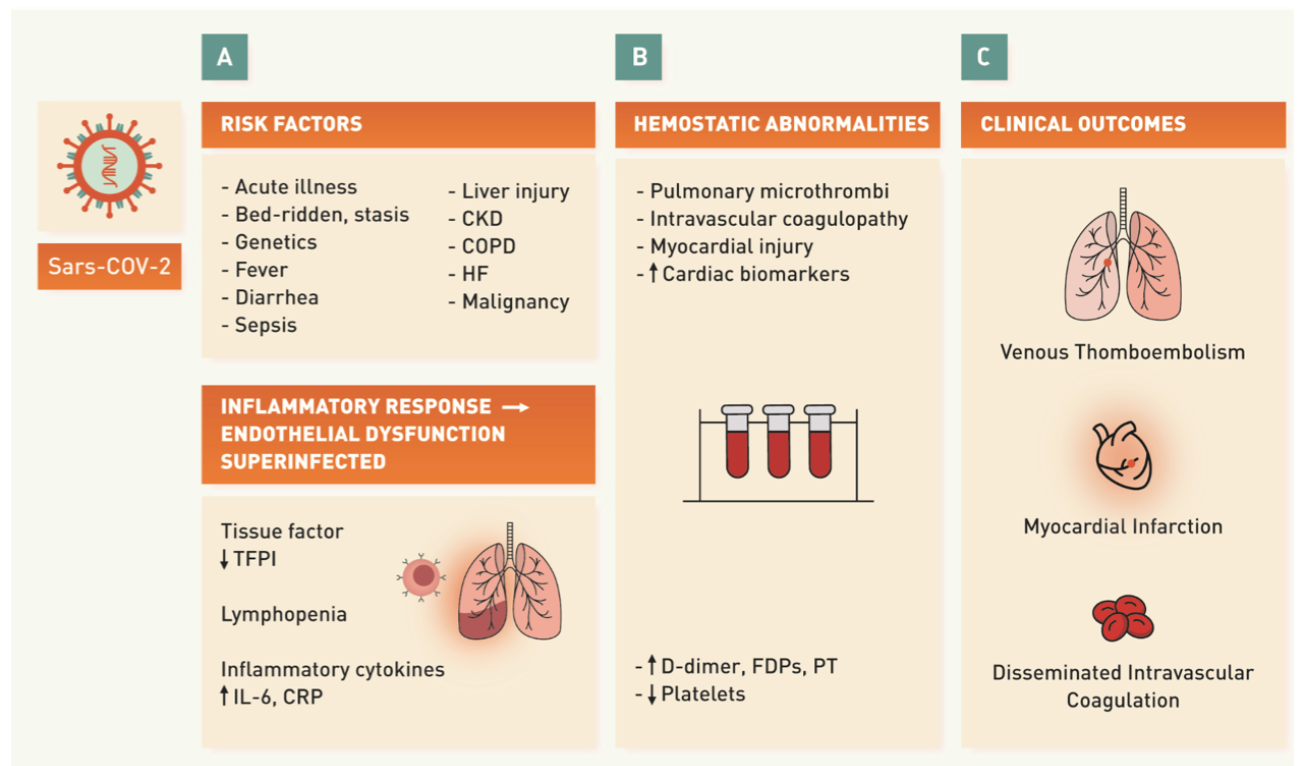
Le COVID-19, maladie virale causée par un nouveau coronavirus, le SARS-CoV-2, est considérée comme une pandémie par l’Organisation mondiale de la santé(1-3). L’Europe, et particulièrement la France, est fortement touchée. La pandémie de COVID-19 peut affecter de plusieurs manières la prévention et la prise en charge des maladies thrombotiques, et thromboemboliques artérielle ou veineuse.
Premièrement, les manifestations cliniques du COVID-19, parmi lesquelles l’hypoxie, peuvent prédisposer les patients à des événements thrombotiques. Les premières données chinoises ont suggéré l’existence de troubles de l’hémostase, pouvant aller jusqu’à une pseudo-coagulation intravasculaire disséminée (CIVD) chez les patients COVID-19(4,5). L’intensité de la réponse inflammatoire et la gravité du COVID-19 peuvent prédisposer à des événements thrombotiques, comme ce fut le cas lors de précédentes flambées de coronavirus zoonotiques(6,7). Deuxièmement, certaines thérapeutiques actuellement à l’essai dans le COVID-19 peuvent avoir des interactions avec les traitements antiagrégants et anticoagulants. Troisièmement, la pandémie, en raison de l’allocation des ressources ou des recommandations de distanciation sociale, peut avoir un effet collatéral négatif sur la qualité des soins des patients qui ne sont pas atteints de COVID-19 mais qui présentent des événements thrombotiques. Dans cette revue, nous allons nous centrer sur la maladie veineuse thromboembolique (MTEV) au cours du COVID-19.
Incidence de la MTEV au cours du COVID-19
Peu d’études ont rapporté l’incidence de la MTEV chez les patients atteints de COVID-19. Dans une étude rétrospective réalisée en Chine, parmi 81 patients atteints d’une forme grave de COVID-19 et admis aux soins intensifs, 20 patients (25 %) ont développé une MTEV aiguë ; aucun des patients n’avait reçu de prophylaxie contre la MTEV(8). Dans une étude portant sur 184 patients atteints de forme grave de COVID-19 et provenant de 3 centres médicaux universitaires aux Pays-Bas, les auteurs ont rapporté que 31 % (IC95% [20-41]) des patients ont développé une MTEV aiguë. Tous les patients avaient reçu une prophylaxie pharmacologique, bien qu’un sous-dosage ait été observé dans 2 des 3 centres participants(9). Il est probable que la MTEV soit sous-diagnostiquée chez les patients atteints de COVID-19 graves. En faire le diagnostic est très important, car le SDRA chez les patients atteints de COVID-19 est en soi une étiologie potentielle de vasoconstriction pulmonaire hypoxique, d’hypertension pulmonaire et d’insuffisance ventriculaire droite. Une dégradation supplémentaire du fonctionnement du système vasculaire cardio-pulmonaire due à une EP peut avoir des conséquences extrêmement graves et irréversibles.
Diagnostic de MTEV chez les patients COVID-19
Des niveaux élevés de D-dimères sont fréquents chez les patients atteints de COVID-19(10), et ne justifient pas un dépistage systématique de MTEV aiguë en l’absence de manifestations cliniques. Un examen de dépistage doit être rapidement envisagé en cas de symptômes typiques de thrombose veineuse profonde (TVP), d’hypoxémie disproportionnée par rapport à l’atteinte pulmonaire du COVID et aux pathologies respiratoires préexistantes ou d’apparition d’une dysfonction ventriculaire droite aiguë inexpliquée. Un défi diagnostique se pose chez les patients atteints de COVID-19, car les examens d’imagerie utilisés pour diagnostiquer une TVP ou une EP peuvent être d’accès difficile en raison du risque de transmission de l’infection à d’autres patients ou au personnel de santé, ou en raison de l’instabilité du patient. Les examens d’imagerie peuvent s’avérer difficiles à réaliser dans le cas de patients atteints d’un SDRA grave qui doivent être placés en décubitus ventral. La détérioration de la fonction ventriculaire droite dans ce contexte peut s’avérer critique ; l’échocardiographie permet la recherche d’une éventuelle aggravation d’une dysfonction ventriculaire droite et, dans de rares cas, la présence d’un thrombus dans l’artère pulmonaire(11).
Modalités thérapeutiques de la MTEV au cours du COVID-19
La sélection d’un traitement lors de l’anticoagulation curative nécessite la prise en compte de comorbidités telles que l’insuffisance rénale ou hépatique, la thrombocytopénie et la fonction d’absorption digestive ; le traitement peut changer au cours de l’hospitalisation jusqu’au moment de la sortie de l’hôpital. Chez de nombreux patients atteints de MTEV, l’anticoagulation parentérale (par exemple, l’héparine non fractionnée [HNF]) est préférable car elle peut être temporairement suspendue et n’a pas d’interaction médicamenteuse connue avec les traitements à l’essai pour le COVID-19. Cependant, les préoccupations concernant l’HNF comprennent le temps nécessaire pour obtenir un TCA à l’objectif thérapeutique et l’exposition accrue au SARS-CoV-2 des soignants pour les prises de sang fréquentes. Les héparines de bas poids moléculaire (HBPM) peuvent être préférées chez les patients pour qui une suspension du traitement pour une procédure invasive est peu probable. Les avantages des anticoagulants oraux directs (AOD) comprennent l’absence de surveillance biologique, la facilité des prises et la gestion des patients ambulatoires. Le risque potentiel (en particulier dans un contexte de défaillance d’organe) peut inclure la détérioration clinique et le manque de disponibilité dans certains centres d’un antidote efficace. Pour les patients sortants, les AOD et les HBPM doivent être privilégiés pour éviter les contacts avec le personnel soignant pour les contrôles d’INR qui sont obligatoires avec les AVK.
Quid d’une anticoagulation efficace empirique sans diagnostic de MTEV ? Compte tenu des troubles de l’hémostase évoqués ci-dessus et des observations relatives à des maladies virales antérieures(12), certains cliniciens utilisent l’anticoagulation parentérale thérapeutique à dose intermédiaire ou à dose complète (plutôt que prophylactique) pour les patients atteints de COVID-19(13), en émettant l’hypothèse qu’elle pourrait conférer un bénéfice pour prévenir les thromboses microvasculaires. Cependant, les données existantes sont très limitées, basées sur une analyse de sous-groupe (n = 99) d’une seule étude rétrospective avec un contrôle limité des facteurs de confusion potentiels, suggérant qu’un score de Sepsis Induced Coagulopathy (SIC) de l’ISTH (incluant la numération plaquettaire, le score de SOFA de défaillance d’organe et le TP-INR) ≥ 4 et des D-Dimères > 6N, seraient statistiquement associés à une réduction de la mortalité à 28 jours, chez des patients COVID pris en charge en réanimation, traités par 7 jours ou plus d’HPBM(14). Une autre étude chinoise rétrospective sur 81 patients avec un COVID grave hospitalisés en réanimation, suggère que des D-dimères > 1 500 ng/ml ont une sensibilité de 85,0 % et une spécificité de 88,5 % pour la détection des événements TEV(15). Ces 2 études suggèrent qu’une anticoagulation efficace serait bénéfique dans un sous-groupe de patients à haut risque (selon le score de SIC et le taux de D-Dimères). La dose optimale chez les patients atteints de COVID-19 grave reste inconnue et justifie des études prospectives. La majorité des experts recommande jusqu’à présent l’anticoagulation prophylactique, bien qu’une minorité considère que la dose intermédiaire ou la dose thérapeutique sont raisonnables.
Les équipes spécialisées dans la prise en charge des embolies pulmonaires permettent d’offrir des soins multidisciplinaires aux patients à risque intermédiaire et élevé de MTEV(16,17-19). Pendant la pandémie COVID-19, ces centres experts doivent passer de l’évaluation clinique des patients hospitalisés à des consultations par téléphone ou par télémédecine. Une EP récurrente malgré une anticoagulation optimale, ou une MTEV cliniquement significative dans le cadre de contre-indications absolues à l’anticoagulation seraient parmi les rares scénarios dans lesquels la mise en place d’un filtre cave pourrait être envisagée(20). Même après la mise en place du filtre cave, l’anticoagulation doit être reprise dès que possible, ce qui est souvent fait en augmentant progressivement les doses et en surveillant étroitement la survenue de saignement. Pour les stratégies de reperfusion en cas d’EP aiguë, il convient de suivre les recommandations des sociétés savantes préexistantes. Les patients à risque intermédiaire hémodynamiquement stables (EP à risque intermédiaire-faible, ou EP à risque intermédiaire-élevé selon la classification ESC)(16,21-23) doivent être pris en charge dans un premier temps par une anticoagulation efficace et une surveillance étroite. En cas de détérioration ultérieure, il faut envisager une fibrinolyse systémique de secours, avec comme alternative des thérapeutiques invasives par cathétérisme de l’artère pulmonaire. Pour les patients présentant une instabilité hémodynamique manifeste (EP à haut risque selon la classification ESC)(16,21-23), une fibrinolyse systémique est indiquée, les traitements par cathétérisme étant réservés aux scénarios dans lesquels la fibrinolyse systémique n’est pas réalisable. La mise en place d’une oxygénation par membrane extracorporelle (ECMO) au lit du malade est préférable dans les cas de positivité au COVID-19 connue ou de suspicion d’infection, plutôt que des stratégies nécessitant l’utilisation d’un laboratoire de cathétérisme ou d’un bloc opératoire(24).
La grande majorité des patients atteints de TVP aiguë symptomatique doivent être traités par anticoagulation efficace, avec un traitement à domicile si possible. Les quelques patients qui peuvent nécessiter des techniques endovasculaires aiguës (fibrinolyse locale ou embolectomie) sont ceux qui présentent une phlébite ou des symptômes véritablement réfractaires(25).
Stratification du risque de MTEV chez les patients COVID-19
Pendant l’hospitalisation pour une pathologie aiguë, y compris pour une pneumonie, les patients présentent un risque accru de MTEV(16,26) et l’anticoagulation prophylactique permet de réduire ce risque(27-31). Plusieurs outils de stratification du risque sont disponibles pour l’évaluation du risque de MTEV dans ce contexte (par exemple, les modèles Caprini, IMPROVE et Padua)(32-37). Une étude récente chinoise, utilisant le modèle de Padoue, a rapporté que 40 % des patients hospitalisés avec un COVID-19 étaient à haut risque de MTEV(38). Tous les patients hospitalisés pour un COVID-19, présentant une insuffisance respiratoire ou des comorbidités (cancer actif, insuffisance cardiaque)(62), les patients alités et ceux nécessitant des soins intensifs doivent bénéficier d’une prophylaxie de la MTEV, sauf contre-indication(39-41). L’OMS recommande une prophylaxie quotidienne par HBPM ou biquotidienne par une HNF sous-cutanée(42). Si la prophylaxie pharmacologique est contre-indiquée, la prophylaxie mécanique (par compression pneumatique intermittente) doit être envisagée chez les patients immobilisés(42,43). L’oubli de doses de la prophylaxie pharmacologique est courant et est probablement associé à un pronostic plus péjoratif(44). Le schéma posologique en une seule prise quotidienne d’HBPM peut être avantageux par rapport à l’HNF en 2 prises. Le risque de MTEV chez les patientes enceintes atteintes de COVID-19 mérite une attention particulière dans une situation déjà à risque(45-47).
Après la sortie de l’hôpital pour une pathologie aiguë, la prolongation de la prophylaxie avec une HBPM(48) ou des AOD(49-52) peut réduire le risque de MTEV, au prix d’une augmentation des saignements, y compris des saignements majeurs(53,54). Bien qu’aucune donnée spécifique au COVID-19 ne soit disponible, il est souhaitable d’employer une stratification individualisée du risque hémorragique et thrombotique, et de considérer une prophylaxie prolongée (jusqu’à 45 jours) pour les patients présentant un risque élevé de MTEV (par exemple, mobilité réduite, comorbidités telles que cancer actif et pour certains auteurs, des D-dimères > 2 fois limite supérieure de la normale) avec un faible risque de saignement(52,55,56). Le rôle de la thromboprophylaxie pour les patients en quarantaine à domicile pour une atteinte non grave de COVID-19 mais avec des comorbidités importantes, ou pour les patients sans COVID-19 qui sont moins actifs en raison de la quarantaine est incertain. La prophylaxie pharmacologique doit être réservée aux personnes les plus à risque, surtout celles à mobilité réduite et ayant des antécédents de MTEV ou une tumeur maligne active.
Modifications des paramètres de l’hémostase au cours du COVID-19
Les anomalies de l’hémostase les plus importantes avec le COVID-19 comprennent des thrombocytopénies(57) et des taux élevés de D-dimères(58). Elles ont été associées à un risque accru de ventilation mécanique, d’admission en unité de soins intensifs (USI), et de décès. Les données concernant les autres paramètres de la coagulation sont souvent contradictoires(59,60). La gravité de la maladie a été associée à un allongement du temps de prothrombine (TP) et de l’INR(61-63), du temps de thrombine (TT)(64), et à un raccourcissement du TCA(61,65-67). Récemment, Tang et coll.(68) ont évalué 183 patients COVID-19, dont 21 (11,5 %) compliqués de décès. Parmi les différences notables entre les patients décédés et ceux qui ont survécu on note des niveaux plus élevés de D-dimères et de produits de dégradation de la fibrine (PDF) (augmentation de 3,5 et 1,9 fois, respectivement) et un allongement du TP (de 14 %). De plus, 71 % des patients atteints de COVID-19 et décédés avaient des critères de CIVD selon les critères définis par l’International Society on Thrombosis and Haemostasis (ISTH)(69), contre seulement 0,6 % chez les survivants. Ces troubles de l’hémostase concourent à certaines formes de coagulopathie qui peuvent prédisposer à la survenue d’événements thrombotiques (Illustration centrale).
Néanmoins, on ne sait pas encore si ces troubles de l’hémostase sont un effet spécifique du SARS-CoV-2 ou une conséquence de l’orage cytokinique qui entraîne un syndrome de réponse inflammatoire systémique (SIRS), comme observé dans d’autres maladies virales(70,73). Les troubles de l’hémostase observés pourraient être liés à une dysfonction hépatique(74). Une étude récente a décrit trois cas de maladie sévère à COVID-19 avec infarctus cérébral, associé à une ischémie bilatérale des membres, dans un contexte d’anticorps antiphospholipides élevés, mais d’isotype IgA et sans lupus anticoagulant(75).
Plusieurs traitements sont actuellement à l’essai dans le COVID-19, en particulier pour les patients qui développent une maladie grave. Certains de ces médicaments ont des interactions cliniques importantes avec les anticoagulants. Certains de ces traitements à l’essai ont été associés à un sur-risque ou à un risque diminué d’événement thrombotique ou de thrombocytopénie dans des études antérieures sur des populations non COVID-19. Par exemple, le bévacizumab, un anticorps monoclonal qui se lie au facteur de croissance endothélial vasculaire (VEGF) est associé à un risque accru de MTEV(76,77). L’hydroxychloroquine, qui a récemment obtenu une autorisation d’urgence d’utilisation par la Food and Drug Administration (FDA) aux États-Unis pour le traitement du COVID-19, a des propriétés antithrombotiques, en particulier sur les anticorps antiphospholipides(78). Le lopinavir/ritonavir peut affecter le choix et la posologie de certains anticoagulants. Les antagonistes de la vitamine K, l’apixaban, et le bétrixaban peuvent nécessiter un ajustement de dose, tandis que l’edoxaban et le rivaroxaban ne doivent pas être administrés. Le tocilizumab, un inhibiteur de l’IL-6, ne nécessite aucun ajustement de la dose d’anticoagulant. Il n’y a pas d’interaction médicamenteuse majeure entre les traitements actuellement à l’essai du COVID-19 et les anticoagulants injectables.
Gestion des anticoagulants au cours des formes graves de COVID-19
Le risque de MTEV, qui est accru chez les patients atteints de maladies graves, est probablement encore plus élevé chez les patients atteints de formes graves du SARS-CoV-2. En soins intensifs, les troubles de l’hémostase, l’immobilité, un état inflammatoire systémique, la ventilation mécanique et les cathéters veineux centraux contribuent au risque de MTEV(79-81). Les carences nutritionnelles et l’insuffisance hépatique peuvent interférer avec la production de facteurs de coagulation(82). Les altérations de la pharmacocinétique en raison de facteurs liés à l’absorption, au métabolisme et à l’élimination rénale (ou hépatique) de ces médicaments dans le cadre d’une défaillance d’organe chez les patients graves peuvent nécessiter un ajustement de la dose d’anticoagulant(83). L’anticoagulation parentérale est recommandée dans la plupart des cas où un traitement anticoagulant est nécessaire pour une maladie thrombotique connue. L’HNF peut être utilisée chez les patients pour qui un acte invasif est envisagé ou dont la fonction rénale se détériore. Si aucune procédure invasive urgente n’est prévue, les HBPM sont une alternative raisonnable(84). Chez les patients nécessitant une ECMO, une anticoagulation est souvent nécessaire pour maintenir la perméabilité du circuit, en particulier en cas de faible débit. Les taux de complications sont inconnus chez les patients atteints du SARS-CoV-2, mais dans d’autres populations souffrant d’insuffisance respiratoire les taux de thrombose et d’hémorragie peuvent atteindre respectivement 53 % et 16 %(85). Les données limitées disponibles sur les résultats de l’ECMO chez les patients atteints du SARS-CoV-2 suggèrent un très mauvais pronostic, avec 5 décès sur 6 patients dans une série et 3 sur 3 dans une autre(86,87). Les données actuelles sont insuffisantes pour recommander des cibles d’anticoagulation pour les patients atteints de COVID-19 nécessitant une ECMO(88).
Le diagnostic de CIVD peut être établi en utilisant le calculateur de score de CIVD de l’ISTH(69). Le suivi régulier en laboratoire de la numération plaquettaire, du temps de Quick, des D-dimères et du fibrinogène chez les patients atteints de COVID-19 est important pour diagnostiquer une aggravation de la coagulopathie. Les surinfections bactériennes doivent être traitées de manière agressive. La prophylaxie par HBPM peut diminuer la production de thrombine et modifier l’évolution de la CIVD. Les résultats préliminaires, bien que le nombre d’événements soit faible et l’adaptation limitée, suggèrent une réponse favorable de la prophylaxie par HBPM sur la CIVD(68,89). Les agents antiplaquettaires à action prolongée doivent être arrêtés chez la plupart des patients atteints de CIVD, sauf s’ils s’avèrent indispensables (par exemple, en cas de SCA récent et/ou d’implantation d’un stent). Pour les patients présentant un COVID-19 modéré ou sévère et une indication à une bithérapie antiplaquettaire (par exemple, angioplastie coronaire au cours des trois derniers mois ou IDM récent) avec une CIVD suspectée ou confirmée sans hémorragie manifeste, les décisions relatives au traitement antiplaquettaire doivent être discutées au cas par cas. En général, il est raisonnable de poursuivre la bithérapie antiplaquettaire si les plaquettes ≥ 50 000/mm3, de le réduire à un seul traitement antiplaquettaire si le nombre de plaquettes est entre 25 000 et 50 000/mm3, et de l’arrêter si le nombre de plaquettes est < 25 000/mm3. Ces propositions peuvent être révisées à la hausse ou à la baisse en fonction du risque relatif individualisé de complications thrombotiques liées au stent par rapport au risque hémorragique.
Les saignements cliniquement manifestes sont rares dans le cadre du COVID-19. Si une hémorragie se produit dans le cadre d’une CIVD associée au COVID-19, la transfusion de produits sanguins doit être envisagée comme pour toute coagulopathie septique(90).
Les principaux piliers de la transfusion de produits sanguins sont :
– (i) concentrés plaquettaires pour maintenir une numération plaquettaire > 50 × 109/l chez les patients atteints de CIVD avec saignement ou > 20 × 109/l chez les patients présentant un risque élevé de saignement ou nécessitant des procédures invasives ;
– (ii) du plasma frais congelé (PFC) (15-25 ml/kg) chez les patients présentant des saignements actifs avec un temps de Quick allongé et/ou un rapport TCA > 1,5 fois la normale ou une diminution du fibrinogène (< 1,5 g/l) ;
– (iii) du concentré de fibrinogène ou du cryoprécipité chez les patients présentant une hypofibrinogénémie grave persistante (< 1,5 g/l) ; – (iv) du concentré de complexe prothrombinique (CCP) si la transfusion de PFC n’est pas possible. L’acide tranexamique ne devrait pas être utilisé en routine dans les CIVD associées à COVID-19.
En pratique
- Les événements liés à une MTEV sont particulièrement fréquents au cours du COVID-19, notamment dans les formes sévères de la maladie.
- Les mécanismes physiopathologiques sous-jacents sont incomplètement élucidés.
- Certaines particularités thérapeutiques doivent être connues pour optimiser la prise en charge.
Figure 1. Mécanismes potentiellement impliqués dans la coagulopathie et la thrombose dans le COVID-19.
A. Le SARS-CoV-2 active la réponse inflammatoire, entraînant la libération de médiateurs de l’inflammation. S’ensuivent l’activation endothéliale et hémostatique, avec une baisse des taux de TFPI et une augmentation du taux de facteur tissulaire. La réponse inflammatoire à une infection sévère induit une thrombopénie et une lymphopénie. Les lésions hépatiques peuvent entraîner une baisse de production des facteurs de la coagulation et une augmentation du taux d’antithrombine.
B. Le COVID-19 peut entraîner des troubles de l’hémostase et une augmentation de la troponine.
C. Un état d’hypercoagulabilité peut aboutir aux événements thromboemboliques veineux, infarctus du myocarde, et une coagulation intravasculaire disséminée.
D’après Bikdeli B et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol 2020 Apr 15.
Cardiologie Pratique : publication avancée en ligne.
*1 Université Paris VII, Denis Diderot ; INSERM UMRS 942 ; Paris ; AP-HP, Hôpital Lariboisière, Département de cardiologie, Paris
2 Sorbonne Universités, UPMC Univ Paris 06, UMR 7211, and Inflammation-Immunopathology-Biotherapy Department (DHU i2B), F-75005, Paris ; INSERM, UMRS 959, F-75013, Paris ; CNRS, FRE3632, Paris ; AP-HP, Groupe Hospitalier Pitié-Salpêtrière, Department of Internal Medicine and Clinical Immunology, Paris