Infeciologia
Aged 40+ With Diabetes Hit Badly by COVID-19, Should Be Vaccine Priority
People with type 2 diabetes as young as 40 years of age face a disproportionately increased risk of dying from COVID-19 infection, indicates a UK analysis of three large-scale datasets that shines a light on the need to prioritize vaccinations in younger vulnerable patient groups.
The research was published February 8 in the journal Diabetologia.
The majority of European countries have prioritized COVID-19 vaccinations for people with type 2 diabetes, but typically only at age 50 and older. However, the data from the current study suggest that this age limit should be lowered.
“It’s important to remember the risk to middle-aged people with diabetes of dying from COVID-19 is very low in absolute terms compared with the elderly,” said lead researcher Andrew P. McGovern, MD, of Royal Devon & Exeter Hospital, Exeter, United Kingdom, in a press release from his institution.
However, he said that “strategies to define priority groups for vaccination must consider the disproportionate relative risk of COVID-19 mortality in middle-aged people with type 2 diabetes whose COVID-19 risk is already elevated by their age.”
McGovern told Medscape Medical News that the magnitude of the effect of type 2 diabetes on COVID-19 deaths “is really what’s surprising” about these new findings, and “not what you would expect.”
He said it is therefore crucial that people with diabetes are put “into the queue” for the vaccine “in the right place, and obviously in countries where the vaccine rollout will be slower, it is more important.”
Bridget Turner, director of policy campaigns and improvement at Diabetes UK, which funded the study, said the results give “important new insights into how much type 2 diabetes adds to the overall risk of dying from coronavirus at different ages, particularly the additional risk that the condition adds in middle-age.”https://c275ced267b81d32d592c3471da53348.safeframe.googlesyndication.com/safeframe/1-0-37/html/container.html
“The UK has made good progress on prioritizing those who are most vulnerable for vaccination, which includes all adults with diabetes,” she added in the press release, “but we need to continue to work at pace to identify and protect those individuals at higher risk.”
Relationship Between COVID Death and Diabetes Is Complex
The authors note that the relationship between COVID-19-related mortality and type 2 diabetes is not simply an “additive effect of diabetes and age-related risk” but appears to be a “more complex” association, with a “disproportionately higher excess relative mortality risk in younger people with diabetes.”
To investigate this, they examined data from two UK population-based studies that had previously reported age-specific hazard ratios for COVID-19 mortality associated with diabetes:
- OpenSAFELY, which included 17.2 million people, of whom 8.8% had diabetes, and had an overall 90-day mortality rate of 0.06%
- QCOVID, comprising 6 million individuals, of whom 7% had diabetes, and had an overall 97-day mortality rate of 0.07%.
The team also looked at data on type 2 diabetes patients with severe COVID-19 from the COVID-19 Hospitalisation in England Surveillance System (CHESS), which contained 19,256 patients admitted to critical care in England, of whom 18.3% had diabetes.
The 30-day in-hospital mortality rate in this study was 26.4%.
They translated the mortality hazard ratios associated with COVID-19 infection in people with diabetes into a “COVID-age,” which equates to the additional years of “death risk” added to an individual’s chronological age if diabetes is present.https://c275ced267b81d32d592c3471da53348.safeframe.googlesyndication.com/safeframe/1-0-37/html/container.html
Taking the QCOVID dataset as an example, the results showed that the “COVID-age” associated with diabetes for someone aged 40 years was 20.4 years; that would indicate that their “mortality risk [for COVID-19] is similar to that of a 60-year-old person without diabetes.”
The impact of diabetes on the COVID-19 death risk decreased with increasing age, such that a diabetes patient aged 50 years had a COVID-age of 16.4 years. This fell to 12.1 years in someone aged 60, and 8.1 years in someone 70 years of age, which means the latter has the same risk of death from COVID-19 as someone without diabetes who is 78.
Similar results were obtained when the team looked at data from the OpenSAFELY study.
But when they looked at the effect of diabetes on COVID-19 mortality risk in the CHESS dataset, it was less pronounced.
Just Looking at Diabetes Is Oversimplistic, but It’s an Easy Marker for Vaccination
The researchers acknowledge that “considering only age and diabetes status when assessing COVID-19-associated risks…is an oversimplification,” as factors such as body mass index (BMI), diabetes duration, and glycemic control are also known to play a role.
However, they say consideration of these factors is “not practical for population-level vaccine rollout.”
“The time-critical nature of population COVID-19 vaccination necessitates pragmatic group-level prioritization, which is the approach initiated by governments thus far,” the team concludes.
This study was supported by Diabetes UK. Study author John M. Dennis is supported by an Independent Fellowship funded by Research England’s Expanding Excellence in England (E3) fund and by the NIHR Exeter Clinical Research Facility. McGovern is supported by the NIHR Exeter Clinical Research Facility.
Diabetologia. Published online February 8, 2021. Full text
Medscape Medical News © 2021
Cite this: Aged 40+ With Diabetes Hit Badly by COVID-19, Should Be Vaccine Priority – Medscape – Feb 15, 2021.TOP PICKS FOR YOU
Túnel carpiano agudo y parálisis nerviosa: patologías de la mano no descritas en pacientes covid
Una serie de pacientes sugiere que el SARS-CoV-2 causaría compresiones nerviosas agudas en los nervios mediano y cubital asociadas a problemas en la mano

Anosmia, ageusia, parálisis facial y óculo-motora… el ‘rastro’ que deja el nuevo coronavirus en el sistema nervioso periférico, una vez resuelta la infección, parece no tener fin. Cada día se descubre una nueva patología asociada a esta infección viral. Para el traumatólogo Juan González del Pino, experto en cirugía de la mano, muñeca y microcirugía dela Policlínica Nuestra Señora del Rosario, en Ibiza, el virus también puede afectar los nervios de la mano, produciendo desde una parálisis de instauración rápida a un síndrome del túnel carpiano agudo.
Así lo ha constatado en al menos ocho pacientes atendidos recientemente. Todos habían superado una infección por SARS-CoV-2, la mayoría con síntomas leves, como pérdida de olfato o gusto, síntomas catarrales, fiebre o dolores musculares. Sin embargo, todos terminaron al cabo de unos meses de la enfermedad con neuropatías localizadas en el codo y la mano.
El cirujano describe cuándo empezó a darse cuenta de la posibilidad de esta nueva patología: “En octubre de 2020, atendí a un hombre de 72 años con atrofia de la musculatura de ambas manos que se había producido en un brevísimo periodo de tiempo. Tenía una parálisis completa bilateral del nervio cubital, responsable de la sensibilidad de los dedos anular y meñique, del movimiento fino y preciso de los dedos, y de parte de las funciones de la pinza del pulgar. Además tenía un síndrome del túnel carpiano en ambas manos de inicio después de su enfermedad. Me aseguraba que hacía 3-4 meses no tenía esas deformidades, ni atrofias y que utilizaba las manos con total normalidad. Al preguntarle sobre enfermedades previas o actuales me indicó que había sufrido infección por coronavirus. Empecé a relacionarlo con la situación clínica y descubrí que había una causa-efecto entre el covid-19 y las neuropatías que el paciente presentaba”.
La historia se repitió en noviembre con varias mujeres de entre 45 y 65 años, que acudieron a su consulta con una evolución idéntica y que igualmente habían estado infectadas por el coronavirus. “El patrón se repetía: enfermedad en la primera ola y desarrollo de atrofias musculares en verano, o enfermedad en la segunda ola y establecimiento de la parálisis entre noviembre y diciembre. Como estos pacientes no tienen dolor, sólo fueron conscientes del problema cuando habían perdido la forma, la destreza y la fuerza de la mano”.
Tras un estudio de esos pacientes con pruebas específicas de conducción nerviosa, constató “un bloqueo agudo y casi completo de la actividad nerviosa en el canal cubital del codo, lo que en términos prácticos se asemejaba a una sección del nervio”.
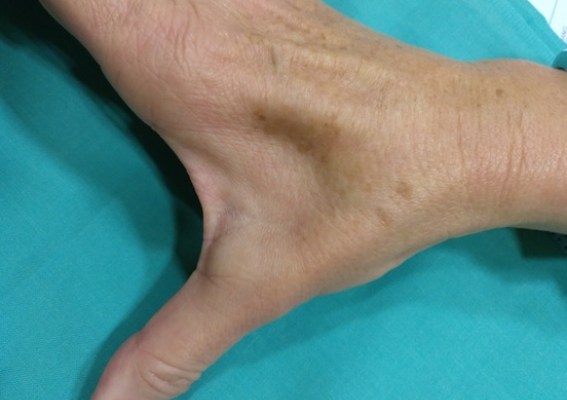
Dada esta rápida evolución y la comprobación del bloqueo casi completo de la actividad nerviosa mediante los estudios específicos, González del Pino optó por una cirugía de liberación de manera inmediata. “Ya he intervenido los primeros pacientes y los hallazgos son sorprendentes: el nervio discurre en el interior de una canal de por sí estrecho, pero pude ver que el ligamento que forma su pared se había retraído, endurecido y lo comprimía, como si hubiera sido estrangulado por una brida apretada al máximo. Se tenía la sensación de que en la zona de máxima compresión el nervio estaba vacío, como si no hubiera nada dentro: los grupos fasciculares que permiten la conducción nerviosa se encontraban extremadamente adelgazados, asemejando un reloj de arena“.
Tras la cirugía de descompresión y transposición del nervio, “incluyendo su vascularización a una zona bajo la musculatura flexo-pronadora de mejores características biomecánicas, en los primeros días los pacientes notaron mejora de la sensibilidad, coordinación de los movimientos finos de la mano y desaparición de las deformidades en garra de los dedos. Aunque no se puede presuponer el éxito a largo plazo, los resultados preliminares son extraordinariamente esperanzadores”, expone.
El especialista opina que probablemente otros colegas en el mundo habrán visto lesiones similares, si bien no hay nada publicado de forma explícita al respecto.
Lo llamativo de estos cuadros es “la velocidad con que se instaura el problema: parálisis producidas en 2-4 meses y no en 10-15 años, que es lo normal cuando el nervio cubital se atrapa en el codo. A pesar de no tener un conocimiento completo de estos problemas en los pacientes con covid-19, la lógica dice que cuanto antes se descomprima el nervio del túnel que lo estrangula, mayores son las posibilidades de recuperación, por eso la cirugía inmediata es, en mi opinión, la única forma de tratamiento: si no se hace rápidamente los músculos atrofiados degeneran, se fibrosan y pierden su capacidad de recuperación”.
Respecto a la evolución a largo plazo, evita hacer afirmaciones rotundas, “porque las toxinas del virus también dañan la fibra nerviosa, pero esta cirugía permite recuperar el riego sanguíneo del nervio y ayudar de forma evidente a su recuperación”.
Síndrome del túnel carpiano agudo
Además de las atrofias musculares debidas a parálisis cubital, González del Pino relaciona ciertos casos del síndrome del túnel carpiano agudo con el Covid, según ha podido observar en varias mujeres de mediana edad. “En este caso no se produce parálisis, sino un dolor repentino e hiperagudo, brusco y violento, que se manifiesta en los dedos pulgar, índice y corazón, y que se incrementa por la noche, impidiendo conciliar el sueño. Algunas ya tenían síntomas leves antes de contraer la infección y se agravaron bruscamente, y otras eran totalmente asintomáticas. En todos los casos el dolor les hace despertarse varias veces, obliga a levantarse, meter las manos en agua fría o caliente y moverlas para mitigar, sólo parcialmente, el dolor. Y así durante semanas. Los estudios de actividad nerviosa reflejaron igualmente afectación grave del nervio mediano a su paso por la muñeca. El denominador común en todas ellas es haber pasado la enfermedad en primavera o después del verano”.
De nuevo se imponía una cirugía inmediata de liberación del nervio, que “produjo la desaparición absoluta e inmediata del dolor y la recuperación completa de la sensibilidad en la primera semana”.
Causas múltiples
En cuanto a la etiopatogenia, González del Pino indica que es multifactorial. No hay apenas bibliografía explícita al respecto, explica, pero “extrapolando la existente a mis casos puedo sugerir como causa probable un proceso de desmielinización focal por inflamación de origen autoinmune, pero no puede descartarse un efecto neurotóxico directo del coronavirus sobre la fibra nerviosa. Determinados nervios (cubital, mediano y ciático-políteo externo) en determinadas zonas estrechas (canal cubital en el codo, túnel carpiano y cabeza del peroné) pudieran ser más susceptibles a fenómenos isquémicos secundarios a vasculitis (afectación endotelial secundaria a “tormenta de citoquinas”), reducción de la microcirculación nerviosa por microtromosis, neuropatía autoinmune o toxicidad directa sobre la fibra nerviosa. Tampoco se puede descartar la posibilidad de neuropatía desmielinizante inflamatoria (“estatus de hiperinflamación e hipercoagulabilidad”). Algunos autores han encontrado parálisis nerviosas de las extremidades por compresión mantenida secundarios a posición prona en pacientes en UVI por distress respiratorio agudo o por hematoma organizado secundario a anticoagulación o edema perineural residual. En estos casos los nervios más afectados son el cubital en el codo y el ciático poplíteo externo en la rodilla. Sin embargo ninguno de nuestros pacientes estuvo ingresado ni recibió tratamiento anticoagulante, por lo que la teoría posicional o hemorrágica puede descartarse”.
Por estos motivos, continúa, parece que la compresión (atrapamiento) se produce de novo en zonas susceptibles por sus especiales características anatómicas (canal cubital en el codo y túnel carpiano), aunque desencadenada por una causa aún no bien dilucidada y probablemente múltiple: respuesta hiperinmune con inflamación, isquemia nerviosa y neurotoxicidad directa a cualquiera de las estructuras nerviosas, vainas o contenido fascicular.
“El agravamiento de nuestros pacientes con síndrome del túnel carpiano también está en la base de estos hechos (edema intracanal y disminución de la microcirculación intraneural del nervio mediano), aunque no existen datos que puedan corroborar una u otra causa”.
Otros efectos músculo-esqueléticos
El cirujano alude a otros posibles efectos de la covid-19 en el sistema músculo-esquelético de los brazos. “He atendido pacientes con “codo de tenista” y diversas tendinitis de la muñeca, así como agravamiento de la enfermedad de Dupuytren”.
Ante los casos descritos, advierte a los pacientes de “que si se despiertan bruscamente con dolor y adormecimiento en las manos (sobre todo por la noche), y a aquellos que noten cómo adelgaza su mano y pierden la habilidad de uso, que acudan al especialista de Traumatología o a un experto en cirugía de la mano”.
Strep A and Tic Worsening: Final Word?

Exposure to Group A streptococcus (GAS) does not appear to worsen symptoms of Tourette syndrome and other chronic tic disorders (CTDs) in children and adolescents, new research suggests.
Investigators studied over 700 children and teenagers with CTDs, one third of whom also had attention deficit hyperactivity disorder (ADHD) and one third who had obsessive-compulsive disorder (OCD).
The youngsters were followed for an average of 16 months and evaluated at 4-month intervals to see if they were infected with GAS. Tic severity was monitored through telephone interviews, in-person visits, and parental reports.
A little less than half the children experienced worsening of tics during the study period, but the researchers found no association between these exacerbations and GAS exposure.
There was also no link between GAS and worsening OCD. However, researchers did find an association between GAS exposure and an increase in hyperactivity and impulsivity in patients with ADHD.
“This study does not support GAS exposures as contributing factors for tic exacerbations in children with CTD,” the authors note.
“Specific work-up or active management of GAS infections is unlikely to help modifying the course of tics in CTD and is therefore not recommended,” they conclude.
The study was published online February 10 in Neurology.https://6ca5e71ac1dd37b9cb45471ee9ba2603.safeframe.googlesyndication.com/safeframe/1-0-37/html/container.html
“Intense Debate”
The association between GAS and CTD stems from the description of Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal infection (PANDAS) — a condition that is now incorporated in the pediatric acute neuropsychiatric syndromes (PANS), the authors note. Tics constitute an “accompanying feature” of this condition.
However, neither population-based nor longitudinal clinical studies “could definitely establish if tic exacerbations in CTD are associated with GAS infections,” they note.
“The link between streptococcus and tics in children is still a matter of intense debate,” said study author Davide Martino, MD, PhD, director of the Movement Disorders Program at the University of Calgary, Calgary, Canada, in a press release.
“We wanted to look at that question, as well as a possible link between strep and behavioral symptoms like obsessive-compulsive disorder and attention deficit hyperactivity disorder,” he said.
The researchers followed 715 children with CTD (mean age 10.7 years, 76.8% male) who were drawn from 16 specialist clinics in nine countries. Almost all (90.8%) had a diagnosis of Tourette syndrome (TS); 31.7% had OCD and 36.1% had ADHD.
Participants received a throat swab at baseline, and of these, 8.4% tested positive for GAS.
Participants were evaluated over a 16- to 18-month period, consisting of:
- Face-to-face interviews and collection of throat swabs and serum at 4-month intervals
- Telephone interviews at 4-month intervals, which took place at 2 months between study visit
- Weekly diaries; parents were asked to indicate in the diary worsening of tics and focus on detecting the earliest possible tic exacerbation.
Beyond the regularly scheduled visits, parents were instructed to report by phone or email any noticeable increase in tic severity and then attend an in-person visit.
Tic exacerbations were defined as an increase of ≥ 6 points on the Yale Global Tic Severity Scale-Total Tic Severity Score (YGTSS-TTS), compared with the previous assessment.
OCD and ADHD symptoms were assessed according to the Yale-Brown Obsessive-Compulsive Scale and the parent-reported Swanson, Nolan, and Pelham-IV (SNAP-IV) questionnaire.
The researchers divided GAS exposures into 4 categories: new definite exposure; new possible exposure; ongoing definite exposure; and ongoing possible exposurehttps://6ca5e71ac1dd37b9cb45471ee9ba2603.safeframe.googlesyndication.com/safeframe/1-0-37/html/container.html
Unlikely Trigger
During the follow-up period, 43.1% (n = 308) of participants experienced tic exacerbations. Of these, 218 participants experienced one exacerbation, while 90 participants experienced two, three, or four exacerbations.
The researchers did not find a significant association between GAS exposure status and tic exacerbation.
Participants who did develop a GAS-associated exacerbation (n = 49) were younger at study exit (9.63 vs. 11.4 years, P < .0001) and were more likely to be male (46/49 vs 210/259, Fisher’s = .035), compared with participants who developed a non-GAS-associated tic exacerbation (n = 259).
Additional analyses were adjusted for sex, age at onset, exposure to psychotropic medications, exposures to antibiotics, geographical regions, and number of visits in the time interval of interest. These analyses continued to yield no significant association between new or ongoing concurrent GAS exposure episodes and tic exacerbation events.
Of the children in the study, 103 had a positive throat swab, indicating a new definite GAS exposure, whereas 46 had a positive throat swab indicating an ongoing definite exposure (n = 149 visits). Of these visits, only 20 corresponded to tic exacerbations.
There was also no association between GAS exposure and OCD symptom severity. However, it was associated with longitudinal changes (between 17% and 21%, depending on GAS exposure definition) in the severity of hyperactivity-impulsivity symptoms in children with ADHD.
“It is known that immune activation may concur with tic severity in youth with CTDs, and that psychosocial stress levels may predict short-term future tic severity in these patients,” the authors write.
“Our findings suggest that GAS is unlikely to be the main trigger for immune activation in these patients,” they add.
Brick or Cornerstone?
Commenting on the study for Medscape Medical News, Margo Thienemann, MD, clinical professor of psychiatry, Stanford University School of Health, Stanford, California, said that in the clinic population they treat, GAS, other pathogens, and other stresses can “each be associated with PANS symptom exacerbations.”
However, these “would not be likely to cause PANS symptoms exacerbations in the vast majority of individuals, only individuals with genetic backgrounds and immunologic dysfunctions creating susceptibility,” said Thienemann, who also directs the Pediatric Acute-Onset Neuropsychiatric Syndrome (PANS) Clinic at Stanford Children’s Health. She was not involved with the study.
In an accompanying editorial, Andrea Cavanna, MD, PhD, honorary reader in neuropsychiatry, Birmingham Medical School, UK, and Keith Coffman, MD, director, Tourette Syndrome Center of Excellence, Children’s Mercy Hospital, Kansas City, Missouri, suggest that perhaps the “interaction of psychosocial stress and GAS infections contributes more to tic exacerbation than psychosocial stress alone.”
“Time will tell whether this study stands as another brick — a cornerstone?— in the wall that separates streptococcus from tics,” they write.
The study was supported by the European Union’s Seventh Framework Program. Martino has received honoraria for lecturing from the Movement Disorders Society, Tourette Syndrome Association of America, and Dystonia Medical Research Foundation Canada; research funding support from Dystonia Medical Research Foundation Canada, the University of Calgary, the Michael P Smith Family, the Owerko Foundation, Ipsen Corporate, the Parkinson Association of Alberta, and the Canadian Institutes for Health Research; and royalties from Springer-Verlag. The other authors’ disclosures are listed in the original article. Cavanna, Coffman, and Thienemann have disclosed no relevant financial relationships.
Neurology. Published online February 10, 2021. Abstract, Editorial
Medscape Medical News © 2021
Cite this: Strep A and Tic Worsening: Final Word? – Medscape – Feb 12, 2021.
As manifestações orais podem nortear o diagnóstico de Covid-19? — Parte 1
A Doença Coronavírus 2019 (Covid-19) se espalhou exponencialmente por todo o mundo desde sua descoberta na China no final de 2019. As manifestações típicas de Covid-19 incluem febre, tosse seca, cefaleia e fadiga. Contudo, apresentações atípicas são cada vez mais relatadas. Estudos reconheceram as lesões orais como manifestações associadas ao Covid-19, sendo que as mais comuns são as ulcerativas, vesicobolhosas e maculares. A ocorrência de manifestações orais no Covid-19 parece ser subnotificada, principalmente devido à falta de exame bucal de pacientes com suspeita e/ou confirmação diagnóstica. O exame oral de todos os casos suspeitos e confirmados é fundamental para melhor compreensão e documentação das manifestações da cavidade oral relacionadas a Covid-19.

Patogenia
Pesquisas atuais sugerem que os danos do novo coronavírus às vias respiratórias e a outros órgãos podem estar relacionados à distribuição de receptores da enzima conversora de angiotensina 2 (ECA-2). Dessa forma, células com receptor ECA-2 podem se tornar hospedeiras do vírus e causar reações inflamatórias em órgãos e tecidos relacionados, como a mucosa da cavidade oral, língua e glândulas salivares. Existem dois mecanismos que podem explicar o desenvolvimento de tais lesões: diretamente, através dos efeitos do vírus replicante, onde essas lesões serão específicas para SARS-CoV-2 e indiretamente, por meio do estresse físico e psicológico associados ao vírus ou secundário aos medicamentos utilizados em seu tratamento.
Manifestações orais relacionadas ao Covid-19
Com o crescente número de casos de Covid-19, vários relatos sobre lesões de cavidade oral têm sido publicados. Assim como nas lesões dermatológicas, a maioria dos trabalhos é de cartas ao editor ou de casos clínicos, portando de baixa qualidade científica. Não há estudos que tenham apresentado lesões patognomônicas, mas a descrição das lesões de cavidade oral já descritas na literatura poderão auxiliar ou nortear os diagnósticos.
Tipos de lesões orais já descritos em pacientes com Covid-19:
Lesões ulcerativas
As lesões ulcerativas são as manifestações orais mais frequentes relacionadas ao Covid-19. A lesões variam entre ulceras únicas, múltiplas, dolorosas ou erosões graves. O local das úlceras varia, mas o dorso da língua é o local afetado com maior frequência, seguido pelo palato duro e a mucosa bucal. Diferentes fatores, incluindo erupção por medicamentos, vasculite ou vasculopatia trombótica secundária a Covid-19, foram sugeridos como outras causas para o desenvolvimento destas lesões (Fig. 1 e 2).


Lesões vesicobolhosas e maculares
As apresentações variam entre bolhas, lesões eritematosas, lesões petequiais e eritema multiformes. Destes, as lesões do tipo eritema multiformes são a apresentação mais comum. A maioria dos casos com manifestações vesicobolhosas e maculares estão associadas a lesões cutâneas (Fig. 3 e 4).

Lesões aftosas
As lesões aftosas se manifestam como múltiplas úlceras superficiais com halos eritematosos e pseudomembranos amarelos e brancos nas mucosas queratinizadas e não queratinizadas. As lesões aftosas sem necrose são observadas em pacientes mais jovens com infecção leve, enquanto lesões aftosas com necrose e crostas hemorrágicas são observadas mais frequentemente em pacientes mais velhos, com imunossupressão e infecção grave. A regressão das lesões orais é associada à melhora da doença sistêmica. O nível elevado de fator de necrose tumoral‐α em pacientes com Covid‐19 pode levar à quimiotaxia de neutrófilos para a mucosa oral e ao desenvolvimento de lesões aftosas. Estresse e imunossupressão secundários à infecção por Covid-19 podem ser outras razões possíveis para o surgimento de tais lesões.
Continua….
Autor(a):
Vera Lucia Angelo Andrade
Graduação em Medicina pela UFMG em 1989 • Residência em Clínica Médica/Patologia Clínica pelo Hospital Sarah Kubistchek • Gastroenterologista pela Federação Brasileira de Gastroenterologia • Especialista em Doenças Funcionais e Manometria pelo Hospital Israelita Albert Einstein • Mestre e Doutora em Patologia pela UFMG • Responsável pelo Setor de Motilidade da Clínica SEDIG BH desde 1995 • http://lattes.cnpq.br/0589625731703512
Em conjunto com: Elias Felipe Romanos da Matta¹.
¹ Especialista em Clínica Médica pelo Hospital Felício Rocho. Especializando em Gastroenterologia e Nutrologia no Hospital Vera Cruz.
Referências bibliográficas:
- Halboub, Esam, Al-Maweri, Sadeq Ali, Alanazi, Rawan Hejji, Qaid, Nashwan Mohammed, & Abdulrab, Saleem. (2020). Orofacial manifestations of Covid-19: a brief review of the published literature. Brazilian Oral Research, 34, e124. Epub October 30, 2020. doi: doi: 10.1590/1807-3107bor-2020.vol34.0124
- Iranmanesh, B, Khalili, M, Amiri, R, Zartab, H, Aflatoonian.(2020). M. Oral manifestations of Covid‐19 disease: A review article. Dermatologic Therapy. e14578. doi: 10.1111/dth.14578
- Anne-Gaëlle Chaux-Bodard, Sophie Deneuve and Aline Desoutter. (2020).Oral manifestation of Covid-19 as an inaugural symptom? J Oral Med Oral Surg, 26 2 18. doi: 10.1051/mbcb/2020011
- Amorim Dos Santos, J., Normando, A., Carvalho da Silva, R. L., De Paula, R. M., Cembranel, A. C., Santos-Silva, A. R., & Guerra, E. (2020). Oral mucosal lesions in a Covid-19 patient: New signs or secondary manifestations?. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases, 97, 326–328. doi: 10.1016/j.ijid.2020.06.012
- Soares, CD, Carvalho, RA, Carvalho, KA, Carvalho, MG, & Almeida, OP (2020). Carta ao Editor: Lesões orais em um paciente com Covid-19. Medicina oral, patologia oral y cirugia bucal , 25 (4), e563 – e564. doi: 10.4317/medoral.24044
- Cruz Tapia, R. O., Peraza Labrador, A. J., Guimaraes, D. M., & Matos Valdez, L. H. (2020). Oral mucosal lesions in patients with SARS-CoV-2 infection. Report of four cases. Are they a true sign of Covid-19 disease?. Special care in dentistry : official publication of the American Association of Hospital Dentists, the Academy of Dentistry for the Handicapped, and the American Society for Geriatric Dentistry, 40(6), 555–560. doi: 10.1111/scd.12520
- Díaz Rodríguez M, Jimenez Romera A, Villarroel M. Manifestações orais associadas com Covid-19. Doenças orais. 2020 Jul. doi: 10.1111/odi.13555
Lengua, palma de la mano y planta del pie, las nuevas “víctimas” de la COVID-19

A un año del primer caso de COVID-19 en España, esta enfermedad mantiene intacta su capacidad de sorprender al colectivo médico. Cada día está más claro que los efectos de la infección provocada por el SARS-CoV-2 van mucho más allá de la pérdida de olfato y los daños pulmonares, como se muestra en un estudio liderado por el Hospital Universitario de la Paz, publicado recientemente en British Journal of Dermatology.[1]
En este estudio con 666 pacientes con COVID-19 ingresados en el hospital de campaña de Ifema entre el 10 y el 25 de abril de 2020 con neumonías leves o moderadas, y cuya primera autora es la Dra. Almudena Nuño-González, dermatóloga del Hospital Universitario de La Paz, se observó que una parte significativa de ellos sufrió lesiones mucocutáneas, orales y palmoplantares.
Este estudio, pionero a nivel mundial, realizado por profesionales del Hospital Universitario de La Paz y de Atención Primaria del Servicio Madrileño de Salud (SERMAS) de la Comunidad de Madrid, sirvió para corroborar las declaraciones previas del Dr. Tim Spector, profesor de epidemiología genética en el King’s College London, en Londres, Inglaterra, que a mediados de enero advirtió que “una de cada cinco personas con COVID-19 presenta síntomas poco comunes que no se incluyen en la lista oficial del Public Health England, como erupciones cutáneas.
Además se está registrando un número creciente de ‘lengua COVID’ y extrañas úlceras bucales”.
Sin embargo, el objetivo de este estudio no era demostrar la veracidad de las afirmaciones del Dr. Spector, ya que estas se realizaron meses después de su finalización.
“Lo que en realidad buscábamos era comprobar cuáles eran las lesiones dermatológicas más frecuentes en pacientes con COVID-19. Dado que en Ifema a todos los pacientes les habían diagnosticado COVID-19, fue un lugar en el que nos resultó fácil conseguir fotografías de las lesiones cutáneas”, explicó la Dra. Pilar Martín-Carrillo, del Consultorio Colmenarejo del SERMAS, participante también en la investigación.
Alteraciones orales en pacientes con COVID-19
El promedio de edad de los 666 pacientes con COVID-19 en los que se realizó el estudio era de 55,67 años, con ligera predominancia femenina (58%) y casi la mitad (47,1%) era de origen latinoamericano; 304 de estos pacientes (45,65%) presentaron una o más manifestaciones mucocutáneas, 78 de ellos (25,65%) padecieron alteraciones en la mucosa oral, incluyendo papilitis lingual transitoria (11,5%), glositis con marcas de los dientes en los laterales (6,6%), úlceras bucales (6,9%), glositis con depapilación en parches (3,9%) y sensación de ardor (5,3%). Todas estas manifestaciones aparecieron generalmente asociadas con disgeusia.
La Dra. Nuño señaló que algunos de estos síntomas podrían estar provocados por factores ajenos al SARS-CoV-2, “como algunos fármacos o la ventilación con oxígeno, que seca la boca y puede irritar la lengua.
Sin embargo, la lengua depapilada es 100% debida a la COVID-19, porque esto no se da por ninguna circunstancia ni ningún tratamiento”, afirmó a Univadis España.
“Este es un hallazgo que puede ayudar al diagnóstico, como la pérdida de olfato o del gusto. Son síntomas muy característicos”, añadió.
Lesiones palmoplantares en pacientes con COVID-19
Por otro lado, también se detectaron alteraciones en la piel de las palmas de las manos y las plantas de los pies en 121 pacientes (39,8%), mayoritariamente descamaciones 25,3% (77 pacientes), manchitas de color rojizo o marrón en 46 de ellos (15,1%) y sensación de ardor conocida como eritrodisestesia en 7% de los pacientes al comienzo de la infección.
Otras manifestaciones observadas fueron urticaria (6,9%), sarpullidos (2,9%) y erupciones vesiculares (1,6%). Tanto la urticaria como los sarpullidos se observaron en cualquier estadio de la enfermedad, mientras que las erupciones vesiculares típicamente aparecieron en los primeros días después de haber comenzado a manifestarse los síntomas. Ambas, la urticaria y las erupciones vesiculares, fueron observadas en los pacientes más jóvenes.
Una variedad de manifestaciones clínicas
La infección por SARS-CoV-2 se ha relacionado con múltiples síntomas: respiratorios, trombóticos, neurológicos, digestivos o cutáneos. Estos últimos se han clasificado en cinco tipos: lesiones acroisquémicas, lesiones vesiculares, erupción urticarial, exantema maculopapular o lesiones livedoides. Sin embargo, hasta la publicación de este trabajo no se habían considerado las alteraciones orales ni las lesiones palmoplantares asociadas a la COVID-19.
Los autores concluyeron que la cavidad oral “se puede alterar por la enfermedad COVID-19. El edema lingual con papilitis lingual transitoria en forma de U o la glositis con depapilación en parches son signos muy característicos, al igual que la sensación de ardor en la cavidad oral. Este ardor puede aparecer también en palmas y plantas con color rojizo o descamación y manchas. Todos pueden ser signos clave para un diagnóstico precoz de esta enfermedad”.
Todos los autores han declarado no tener ningún conflicto de interés económico pertinente.
Medscape Noticias Médicas © 2021 WebMD, LLC
Citar este artículo: Lengua, palma de la mano y planta del pie, las nuevas “víctimas” de la COVID-19 – Medscape – 11 de feb de 2021.
The Challenges of Antiviral Treatments
Physician Claudette Poole doesn’t take long to rattle off a list of antiviral medications she prescribes to her patients. “There really aren’t very many,” says Poole, a pediatric infectious disease physician at the University of Alabama at Birmingham.

And for people with Covid-19, there’s just one approved for use: remdesivir, which doesn’t seem to save lives, but speeds recovery in those who do get well. Clearly, more antivirals would be nice to have — so why don’t we have them? Inventing them, it turns out, is not so easy.
Viruses rely on human cell machinery to copy themselves, so antiviral designers face a challenge: how to stop the virus without damaging the inner workings of healthy cells too. While scientists have found several solutions to the problem, the antiviral pharmacopeia still lags behind the plethora of antibiotics available to treat bacterial infections.
But as researchers build up their knowledge of viral life cycles, antivirals may be poised to catch up. Scientists are also planning for future pandemics, in the hopes of having a better selection of antivirals to try the next time around.
Here’s where antivirals stand today, and how the list might be growing.
How Do Antivirals Work?
An antiviral drug can block any of the steps a virus uses to copy itself. To do its dirty work, a virus must attach to a host cell, sneak inside and trick that cell into copying viral genes and crafting viral proteins; after that, the newly made viruses must escape to infect new targets. At each step, viral genes or proteins need to interact with various host molecules, and each of these interactions offers an opportunity for antiviral drugs. The drugs often mimic those host molecules and act as decoys to interfere with the viral life cycle and reduce its spread.https://4124fa68669c2e6429bf5d79b5331880.safeframe.googlesyndication.com/safeframe/1-0-37/html/container.html
A common approach is to interfere with the copying of viral genes into DNA or RNA to form new viral genomes. Viruses frequently have their own versions of proteins, called polymerases, for this task. The polymerases add individual building blocks called nucleotides, one by one, to the new genome as it’s being built.
For example, the drug acyclovir, used to treat herpes, goes after this genome-copying step. To the virus’s polymerase, the medicine looks like just another building block — but it’s not. Once the decoy gets into the growing strand, it prevents the addition of any more nucleotides. For the virus, it’s game over.
Another drug, oseltamivir (Tamiflu) for influenza, acts at the stage of viral exit from the infected cell. The virus uses a key protein called neuraminidase to dissolve its way out, but oseltamivir sticks to the neuraminidase and stops it from working.
Since antivirals don’t eradicate viruses directly — they just stop them from spreading cell to cell or person to person — it’s up to the body’s immune system, when possible, to mop up the invaders already present. That’s why it’s important to start antiviral treatment early, while viral numbers remain low. “The faster you can take the drug, the more you can limit the virus’s ability to spread,” says virologist Mark Heise of the University of North Carolina at Chapel Hill. Tamiflu, for example, works best when taken within 48 hours of the first symptoms, helping people recover about a day faster.
Why Are There So Few Antivirals?
The number of antivirals is paltry compared with the list of bacteria-fighting antibiotics. That’s due to several factors.
For one, antibiotics were first out of the starting gate, notes Erik De Clercq, an emeritus professor of biomedicine at KU Leuven in Belgium. The first, penicillin, was discovered in 1928 and first used in a patient in 1940. In contrast, the first antiviral, idoxuridine, was developed as an anticancer agent in 1959, was reported to block viruses in 1961, and approved in 1963 to treat herpes infections of the eye. (De Clercq, a leader in early antiviral research, described his scientific journey in the Annual Review of Pharmacology and Toxicology in 2011.)
Plus, viruses are much trickier targets than bacteria, says Monica Gandhi, an infectious disease physician at the University of California, San Francisco. Bacteria are whole living cells with all the metabolic pathways they need for survival, so they offer plenty of targets for attack. They also have unique features such as cell walls that aren’t found in human cells. That means antibiotics can interfere with cell walls, or other bacteria-specific parts and processes, to kill the pathogens without harming our own cells. And because microbes evolved antibiotics to battle each other, there is a diverse array of the compounds out in nature.https://4124fa68669c2e6429bf5d79b5331880.safeframe.googlesyndication.com/safeframe/1-0-37/html/container.html
In contrast, viral pathogens live inside our own cells and depend on our proteins for most of their needs, so they offer no such easy targets. And few natural antivirals exist, so scientists need to invent them from scratch, says Kathie Seley-Radtke, a medicinal chemist at the University of Maryland, Baltimore County.
Moreover, antivirals have a limited number of possible shapes. That’s because, to block a virus’s actions, they must fit into viral proteins as decoys.
The biggest challenge, says Seley-Radtke, is to ensure that the drugs don’t hurt the human hosts as well. For example, in the case of nucleotide mimics like acyclovir, wouldn’t there be a risk that they would get into the cell’s DNA as well as the virus’s?
There are ways around that problem. In the case of acyclovir, the drug that patients swallow is an inactive form, and it is mainly activated by a viral protein. “It targets the virus very nicely, while leaving host cell DNA alone,” says Poole, who reviewed the use of acyclovir and other antivirals for newborns with herpes simplex virus in the 2018 Annual Review of Virology.
There is one unfortunate similarity between antibiotics and antivirals: In both cases, pathogens can make tiny changes to their genes and proteins that leave them unharmed by the drug. “Resistance is a huge issue in antivirals,” says Poole. Doctors used to prescribe drugs called adamantanes to people with the flu, for example — but the influenza viruses circulating among people today are unaffected by the drugs. “They are no longer effective at all,” says Poole. “There’s a desperate need to find other flu antivirals.”
The one exception to the dearth of antivirals is the flourishing pharmacopeia of meds against the human immunodeficiency virus, resulting from decades of research. Gandhi says that she can select from 30 or so medications to treat her HIV-positive patients. More are on the way — and just as well, because HIV can quickly evolve resistance to any one drug.
“HIV research set the tone,” says Heise, and it’s bringing other antivirals in its wake. “We’re seeing, again, new antivirals coming out much more quickly over the last several years.” In the last five years, for example, novel medications have turned hepatitis C from a chronic to curable condition.
“I think we’ll see more drugs for acute viral infections coming forward as well,” says Heise.
How Are Antivirals Being Used Against Covid-19?
Drugs designed against one virus often work against others, because proteins such as the polymerases used to copy virus genomes are similar across a wide range of viruses. But the current scourge requires more than the usual amount of cleverness from antiviral designers.
“Coronaviruses are pretty tricky,” Seley-Radtke says. Simple nucleotide mimics like acyclovir won’t work, because these viruses have another protein that acts as an editor, monitoring the polymerase’s work, recognizing the decoy and cutting it out.
Enter remdesivir, which had already been tested in people with Ebola (though it didn’t help them much). It’s a nucleotide mimic, but it’s a bit special. Once incorporated into a piece of the new viral genome — which in the case of coronaviruses is made of RNA — it doesn’t stop the strand’s growth right away. The polymerase keeps adding normal nucleotides. But after it’s added a few, the drug bends the RNA strand so badly that the polymerase can’t keep building. By then, though, the coronavirus editor protein no longer works; the normal nucleotides added after remdesivir seem to get in the way, says Seley-Radtke. Thus, the polymerase is stuck.
Despite that clever trick, remdesivir’s performance in people with Covid-19 has been decidedly lukewarm. On the plus side: In a trial of 1,062 people hospitalized with the virus, those who were treated with remdesivir recovered more quickly than those who received an inactive placebo. Based on this and two similar studies, the US Food and Drug Administration authorized remdesivir to treat hospitalized patients.
But what remdesivir doesn’t seem to do is save lives. In November, the World Health Organization, citing its own larger but preliminary study, recommended against remdesivir for hospitalized patients for the time being, until more research can be done. The WHO noted “no evidence that remdesivir improves survival and other outcomes.”
The results, which at first blush may seem confusing, make sense considering how antivirals work, says Poole. Because remdesivir requires multiple intravenous infusions, it’s given only to hospitalized patients. But by the time a person with Covid-19 is ill enough to be hospitalized, the virus has already run rampant across their body, so remdesivir comes too late to do much good. “The game-changer,” she says, “is going to be when we find an antiviral that you can give to people orally before they get into the hospital.”https://4124fa68669c2e6429bf5d79b5331880.safeframe.googlesyndication.com/safeframe/1-0-37/html/container.html
Remdesivir’s maker, Gilead Sciences, is working on an inhaled version. And there are other antivirals in the pipeline. For example, Seley-Radtke is optimistic about another nucleotide analog, known as AT527. Under joint development by Roche and Atea, AT527 is now midway through human trials. Like remdesivir, it has delayed action, so it avoids being edited out of a growing RNA strand. But unlike remdesivir, it’s a pill you can swallow. The companies hope it can be used by hospitalized and non-hospitalized patients alike, and perhaps even be taken by people exposed to Covid-19 to prevent infection from taking hold to begin with.
The pandemic has sent scientists scrambling to find treatments. Heise, for one, is testing a wide range of drugs — not just standard antivirals — against SARS-CoV-2 in lab dishes, as part of the Rapidly Emerging Antiviral Drug Discovery Initiative (READDI). The idea is that, because the virus depends on many processes in human cells, a variety of medications that act on human proteins might give doctors an edge by hurting the virus more than the patient. That throws the doors open to considering medications that were originally designed for cancer, psychosis, inflammatory conditions and autoimmune disease, to see if they might have a shot against Covid-19.
But the READDI collaborators — including academic centers, pharmaceutical companies and nongovernmental organizations — are aiming for more than a Covid-19 treatment. READDI hopes to identify and test potential medications for as-yet-unknown infections that may crop up in the future.
By getting early human safety testing done ahead of time, they’ll be ready to spring into action when those future outbreaks happen. As Heise says, “We don’t want to repeat what we’ve just been through.”
Amen to that.
This article is part of Reset: The Science of Crisis & Recovery, an ongoing series exploring how the world is navigating the coronavirus pandemic, its consequences and the way forward. Reset is supported by a grant from the Alfred P. Sloan Foundation.
This article originally appeared in Knowable Magazine on February 9, 2021. Knowable Magazine is an independent journalistic endeavor from Annual Reviews, a nonprofit publisher dedicated to synthesizing and integrating knowledge for the progress of science and the benefit of society. Sign up for Knowable Magazine’s newsletter.123
Knowable Magazine © 2021
Cite this: The Challenges of Antiviral Treatments – Medscape – Feb 11, 2021.
Deficiência de #vitamina D e Covid-19: o que há de novo?

Durante a pandemia, muito se questionou se haveria alguma relação entre a deficiência de vitamina D e Covid-19, principalmente em casos mais graves da infecção. A vitamina D é um hormônio esteroide responsável pela regulação dos níveis corporais de cálcio e sódio e da mineralização óssea. Também exerce importante papel no controle de diversas patologias, como doenças autoimunes e imunossupressão.
A vitamina D é sintetizada no organismo humano pela ingestão de alimentos ricos em proteínas animais e vegetais e pode ser classificada como colecalciferol (D3) e ergocalciferol (D2) respectivamente de acordo com sua origem alimentar. Além disso, é sintetizada pela exposição aos raios ultravioletas (UVB) que através da epiderme transforma um derivado do colesterol (7-DHC) em colecalciferol. No fígado, este é convertido em 25 hidroxi vitamina D que é transformada no seu produto final no rim. A maioria da população adquire vitamina D pela exposição solar, porém a população americana tem seu aporte mais relacionado à alimentação como leite, sucos e cereais fortificados com vitamina D. Populações que habitam altas altitudes e com maior concentração de melanina possuem deficiência natural da vitamina por estarem menos expostas à luz solar.
Uma análise com 4962 pacientes na National Health and Nutrition Examination Survey mostrou que quase 40% da amostra apresentava níveis de vitamina D abaixo de 20ng/ml e estavam mais relacionados à pessoas obesas e com diabetes tipo 1 e 2.
Deficiência de vitamina D e Covid-19
Atualmente alguns estudos vêm correlacionando a deficiência de vitamina D com um maior risco de complicações junto a infecção por Covid-19, principalmente em pacientes obesos, negros e com diabetes tipo 1 e 2. Porém, os estudos têm se mostrado confusos e esparsos como alguns exemplos citados abaixo:
- Um estudo realizado na França com 77 pacientes idosos e diagnosticados com Covid-19, evidenciou que aqueles que consumiam rotineiramente suplementação de vitamina D antes de serem infectados, apresentaram quadros mais leves em comparação com aqueles que tomaram vitamina D logo após o diagnóstico. E um estudo espanhol piloto randomizado com 76 pacientes hospitalizados com Covid-19, que receberam altas doses de vitamina D durante a internação, apresentaram menor incidência de internação em unidade fechada.
- Estudo em um hospital italiano mostrou uma ligação de baixos níveis de vitamina D com um risco aumentado de desenvolvimento de pneumonia por SARS CoV-2. Porém, idade, sexo e comorbidades apresentaram-se como maiores fatores de risco para mortalidade do que a deficiência de vitamina.
- Outro estudo realizado por pesquisadores do JAMA Network Open by University of Chicago observou uma grande conexão de deficiência de vitamina D com maiores chances de testagem positiva para Covid-19.
- Estudo realizado em Julho de 2020 na Indonésia, apesar de não ter sido revisto e publicado, tomou um grande vulto nas redes sociais de forma não oficial, também demonstrando uma forte ligação entre deficiência de vitamina D e maior risco de complicações por Covid-19. Porém, não foi possível analisar nenhum parâmetro desse estudo, o que o tornou desconsiderado.
- Em outubro de 2020, editores do PLoS One, também expressaram uma certa ligação entre níveis deficientes de vitamina D (abaixo de 30ng/ml) com o dobro de chances de óbito por Covid-19.
Todos os estudos demonstrados apresentaram muitas variáveis e bastante conflito de interesses, principalmente relacionados à indústria de suplementação, o que impede que haja uma conclusão assertiva que possa correlacionar efetivamente a deficiência de vitamina D com casos graves de Covid-19. Porém, níveis adequados de vitamina D são essenciais para a saúde óssea e a prevenção da deficiência vitamínica é sempre um objetivo clínico essencial.
Autor(a):
Gabriela Queiroz
Graduação em Medicina pela Universidade do Estado do Rio de Janeiro (UERJ) ⦁ Pós-Graduação em Anestesiologia pelo Ministério da Educação (MEC) ⦁ Pós-Graduação em Anestesiologia pelo Centro de Especialização e Treinamento da Sociedade Brasileira de Anestesiologia (CET/SBA) ⦁ Membro da Sociedade Brasileira de Anestesiologia (SBA) ⦁ Ênfase em cirurgias de trauma e emergência, obstetrícia, plástica estética reconstrutiva e reparadora e procedimentos endoscópicos ⦁ Experiência em trauma e cirurgias de emergência de grande porte, como ortopedia, vascular e neurocirurgia ⦁ Experiência em treinamento acadêmico e liderança de grupos em ambiente cirúrgico hospitalar ⦁ Orientadora acadêmica junto à classe de residentes em Anestesiologia ⦁ Orientadora e auxiliar em palestras regionais e internacionais na área de Anestesiologia.
Referências bibliográficas:
- Rubin R. Sorting Out Whether Vitamin D Deficiency Raises COVID-19 Risk.JAMA. 2021;325(4):329-330.
La exposición prenatal a #terapia antirretroviral puede contribuir a los cambios cardiacos en los bebés

NUEVA YORK, USA. Los bebés no infectados con virus de inmunodeficiencia humana expuestos a la terapia antirretroviral antes del nacimiento pueden tener cambios cardiovasculares “relevantes”, informan médicos en España.[1]
“Se observó deterioro cardiaco subclínico junto con presión arterial más alta y grosor de la íntima media carotídea más grueso en bebés expuestos al virus de inmunodeficiencia humana a los seis meses de edad. La mitad de ellos presentaba hipertensión”, informaron la Dra. Marta López Carbonell y sus colaboradores, de la Universidad de Barcelona, en Barcelona, España.
“Nuestros hallazgos respaldan posible aumento del riesgo cardiovascular en bebés no infectados por el virus de inmunodeficiencia humana que estuvieron expuestos en el útero a terapia antirretroviral”, escribieron en Clinical Infectious Diseases.
Los investigadores dieron seguimiento a 34 bebés no infectados expuestos al virus de inmunodeficiencia humana y a 53 bebés no expuestos hasta los 6 meses de edad. Los esquemas de terapia antirretroviral durante el embarazo incluyeron dos inhibidores nucleosídicos de la transcriptasa inversa (abacavir más lamivudina: 32%; emtricitabina más tenofovir: 41%, y zidovudina más lamivudina: 21%).
A la edad de 6 meses los lactantes no infectados expuestos al virus de inmunodeficiencia humana tenían paredes miocárdicas más gruesas (grosor medio de la pared septal: 5,02 frente a 3,98 mm; p < 0,001) y disfunción sistólica relativa con disminución del desplazamiento del anillo mitral (8,57 frente a 10,34 mm; p = 0,002 ) y disminución de tricúspide S’ (9,71 frente a 11,54 cm/s; p = 0,003) junto con disfunción diastólica relativa indicada por un tiempo prolongado de relajación isovolumétrica izquierda (58,57 frente a 47,94 ms; p < 0,001).
La evaluación vascular reveló presiones arteriales sistólicas (102 frente a 80 mm Hg; p < 0,001) y diastólicas (64 frente a 55 mm Hg; p = 0,045) significativamente más altas. La mitad de los niños expuestos al virus de inmunodeficiencia humana cumplió los criterios de hipertensión, en comparación con 3,77% del grupo no expuesto (p < 0,001).
Los niños expuestos al virus de inmunodeficiencia humana también tenían grosor de la íntima media carotídea medio más grueso (0,62 frente a 0,51 µm; p = 0,015).
“Estos cambios podrían permitirnos distinguirlos como población en riesgo incluso desde la vida fetal, estableciendo seguimiento estricto y más prolongado”, indicaron los autores.https://8726cd39b10dfee9ce799a8c59060cc7.safeframe.googlesyndication.com/safeframe/1-0-37/html/container.html
“Curiosamente, nuestro estudio muestra por primera vez que la hipertensión infantil podría predecirse a partir de la vida fetal mediante la evaluación de los esquemas maternos de terapia antirretroviral que contienen zidovudina o midiendo el espesor de la pared del tabique fetal mediante ecocardiografía fetal”, añadieron.
Los investigadores agregaron que sus hallazgos también “respaldan la tendencia actual de considerar los esquemas de terapia antirretroviral que contienen zidovudina durante el embarazo como alternativa, debido a la dosificación compleja y la asociación con tasas más altas de efectos adversos leves a moderados”.
Por lo tanto, “evitar los esquemas de terapia antirretroviral que contienen zidovudina durante el embarazo, promover hábitos de estilo de vida saludable y evitar otros riesgos cardiovasculares desde la niñez, podrían potencialmente prevenir eventos cardiovasculares más adelante en la vida”.
El estudio no tuvo financiamiento comercial. Los autores han declarado no tener ningún conflicto de interés económico pertinente. La Dra. López no respondió a una solicitud de comentarios al cierre de la edición.
Traducido y adaptado por el equipo de Medscape en español.
Reuters Health Information © 2021
Citar este artículo: La exposición prenatal a terapia antirretroviral puede contribuir a los cambios cardiacos en los bebés – Medscape – 9 de feb de 2021.
Covid-19 durante a gestação pode aumentar risco de hipertensão e pré-eclâmpsia

Dra. Nigel Madden
“Isso não foi totalmente surpreendente, visto que a inflamação vem sendo implicada na patogênese tanto da doença hipertensiva gestacional como da infecção por SARS-CoV-2, portanto, uma pode exacerbar a outra”, a Dra. Nigel Madden, médica residente no Departamento de Ginecologia e Obstetrícia da Columbia University, nos Estados Unidos, disse ao Medscape após apresentar os resultados.
A doença hipertensiva gestacional ocorre em 10% a 15% de todas as gestações e representa a principal causa de morbidade e mortalidade materna e perinatal do mundo, explicou a Dra. Nigel aos participantes do encontro. Embora não esteja claro o que causa as doenças hipertensivas na gestação, de forma geral, “é possível que o estado inflamatório agudo da infecção por SARS-CoV-2 possa incitar ou exacerbar a doença hipertensiva gestacional”, comentou a Dra. Nigel.
Os pesquisadores conduziram uma revisão retrospectiva de prontuários de 1.715 pacientes que tiveram gestação única e que foram submetidas a exame por reação em cadeia da polimerase (PCR, sigla do inglês, Polymerase Chain Reaction), a partir de amostra nasofaríngea, no momento da admissão hospitalar na instituição na qual realizaram o trabalho de parto, entre março e junho de 2020. Os pesquisadores excluíram pacientes com história de hipertensão crônica.
No total, 10% das pacientes apresentaram resultado positivo para covid-19 (N = 167) e 90% apresentaram resultado negativo (N = 1.548). Houve várias diferenças basais entre os grupos. As pacientes com resultado positivo tenderam a ser mais jovens; média de idade de 28 anos versus 31 anos para as pacientes com resultado positivo. A proporção de pacientes acima de 35 anos de idade também foi maior no grupo negativo (P < 0,01). Houve diferenças significativas na composição racial dos grupos; metade do grupo positivo referiu “outras” ao indicar a própria raça. A maior disparidade basal entre os grupos foi em relação ao seguro de saúde: 73% das pacientes com resultado positivo para covid-19 usavam o Medicaid, enquanto apenas 36% das pacientes no grupo negativo para covid-19 usavam esse recurso. As que tinham plano de saúde particular foram mais propensas a apresentar resultado negativo (43%) do que positivo (25%) (P < 0,01).
Os pesquisadores definiram hipertensão gestacional como pressão sistólica ≥ 140 mmHg ou pressão diastólica ≥ 90 mmHg em duas aferições com no mínimo quatro horas de intervalo. O diagnóstico de pré-eclâmpsia exigiu pressão arterial elevada (mesmos critérios usados para definir hipertensão), além de proteinúria, caracterizada por razão proteína/creatinina ≥ 0,3 mg/dL ou proteína ≥ 300 mg na urina de 24 horas. A pré-eclâmpsia grave exigiu alterações laboratoriais específicas, edema pulmonar, sintomas de cefaleia, alterações visuais, dor torácica, dispneia ou dor no quadrante superior direito
Em relação à doença hipertensiva gestacional, a ocorrência da doença foi mais do que duas vezes maior entre as pacientes com covid-19 (17,9%) em comparação com as pacientes que apresentaram resultado negativo (8,4%). O grupo positivo para covid-19 teve probabilidade significativamente maior de apresentar hipertensão gestacional e pré-eclâmpsia sem características de gravidade do que o grupo negativo para covid-19. As taxas de pré-eclâmpsia com características de gravidade não foram significativamente diferentes entre os grupos.
| Negativas para covid-19 | Positivas para covid-19 | Valor de P | |
|---|---|---|---|
| Hipertensão gestacional | 3,6% | 9,0% | < 0,001 |
| Pré-eclâmpsia | 1,3% | 3,6% | 0,03 |
| Pré-eclâmpsia grave | 3,6% | 5,4% | 0,12 |
A gravidade da doença hipertensiva não diferiu entre os grupos. As limitações do estudo incluíram o fato de o desenho ter sido retrospectivo, o pequeno número de pacientes positivas para covid-19 e o estudo ter sido conduzido em uma única instituição na cidade de Nova York, nos EUA. No entanto, a população do estudo era diversificada e a pesquisa foi realizada durante o pico de infecções no epicentro da pandemia de covid-19.
“Esse foi um estudo de grande significado clínico”, disse a Dra. Kim Boggess, médica da University of North Carolina, nos EUA, enquanto moderava a sessão. “Eu diria vocês de Nova York são os mais bem posicionados para responder a algumas questões que precisam ser respondidas a respeito do efeito da infecção pelo coronavírus na gestação”.
A Dra. Kim perguntou se o estudo avaliou associações relacionadas com a gravidade da covid-19. Apenas 10 pacientes eram sintomáticas, disse a Dra. Nigel, e apenas uma apresentou pré-eclâmpsia com características de gravidade.
A Dra. Michelle Y. Owens, médica, professora e chefe de medicina materno-fetal no University of Mississippi Medical Center, nos EUA, que também moderou a sessão, disse que os achados requerem que os médicos atentem para a possibilidade de hipertensão e transtornos hipertensivos ao atenderem pacientes positivas para covid-19.
“Além disso, essas mulheres deveriam ser instruídas em relação aos transtornos hipertensivos e sintomas comuns, a fim de facilitar o diagnóstico precoce e o tratamento, quando indicado”, disse a Dra. Michelle. “Eu acredito que isso é de particular interesse para as mulheres que não são gravemente afetadas pela covid-19, na medida em que essas alterações podem ocorrer enquanto elas estiverem em quarentena ou sendo monitoradas remotamente. Isso aumenta a necessidade de avaliação remota ou monitoramento domiciliar da pressão arterial materna.”
As Dras. Nigel, Kim e Michelle informaram não ter conflitos de interesses.
Encontro anual de 2021 da Society for Maternal-Fetal Medicine (SMFM): Abstract 32. Apresentado em 28 de janeiro de 2021.
Medscape Notícias Médicas © 2021 WebMD, LLC
Citar este artigo: Covid-19 durante a gestação pode aumentar risco de hipertensão e pré-eclâmpsia – Medscape – 9 de fevereiro de 2021.
Origen del SARS-CoV-2: la OMS sospecha de un animal intermedio, y no descarta los alimentos congelados
La OMS, tras una investigación sobre el terreno del primer brote dectado, mantiene la explicación zoonótica de la covid-19, si bien también indagará en un eventual origen en la cadena de frío de alimentos
Como era de prever, las pesquisas para resolver el origen del coronavirus que trae en jaque a toda la humanidad no se han resuelto en cuestión de unos días (el especialista en enfermedades infecciosas Dominic Dwyer ya dijo que probablemente se necesitarán años para comprender la procedencia de la covid-19).
El equipo de científicos de la Organización Mundial de la Salud (OMS) que ha viajado al lugar donde se comunicaron los primeros casos de covid-19, la ciudad china de Wuhan, ha expuesto hoy martes en una larga rueda de prensa los resultados de sus indagaciones. Este grupo de expertos llegó a Wuhan el 14 de enero y, tras la pertinente cuarentana de dos semanas, ha recabado información del mercado de mariscos y pescasos de Huanan, donde se registraron los primeros enfermos conocidos, así como el Instituto de Virología de Wuhan, donde había equipos científicos investigando en coronavirus.
Según los investigadores, hay nueva información, pero nada que dé un vuelco a las conjeturas actuales sobre la aparición del SARS-CoV-2: los murciélagos como reservorio del virus es hoy la sospecha más firme, aunque parece que los animales no estaban necesariamente en Wuhan. Queda prácticamente descartada la hipótesis de que el virus saliera (accidentalmente o por una conspiración) de un laboratorio, incluido el Instituto de Virología de Wuhan.
Sin embargo, sí es probable que de su reservorio natural pasara por un animal intermedio antes de que comenzar a transmitirse entre humanos. Los investigadores de la OMS también han abierto la puerta a la procedencia de otros lugares, por la vía de los alimentos congelados, una línea de investigación impulsada por los expertos chinos, que han comunicado reiteradamente hallazgos de rastros de coronavirus en envases de alimentos importados. Sobre esta última hipótesis, los expertos de la OMS matizan que si bien se sabe que el virus puede sobrevivir ambientes fríos y congelados, no se ha confirmado que pueda transmitirse desde este medio a los humanos.
El jefe de la misión internacional de expertos de la OMS, el especialista en zoonosis danés Peter Ben Embarek, ha asegurado en la rueda que “todo continúa señalando a un reservorio de este virus, o de un virus similar, en poblaciones de murciélagos”.
Ya que en Wuhan y en su región no hay colonias de murciélagos, ha explicado el experto, intentaron encontrar “otras especies animales que pudieron contribuir a introducir el virus” en la ciudad. “No parece que hubiera una gran circulación del virus en ninguna especie animal en el país”, donde los científicos chinos analizaron miles de especies sin que se pudiese identificar a ninguna especialmente portadora.
En el mercado se vendían productos congelados, “algunos importados”, dijo, además de animales salvajes domesticados y productos elaborados con ellos. “Tenemos que seguir esta pista, tanto en los productos congelados como en los semiprocesados y los crudos”. Asimismo, ha destacado que no hay “grandes evidencias” de la transmisión del virus en Wuhan antes de diciembre de 2019 y que los primeros casos en la ciudad no se dieron solo en el mercado de mariscos y pescados, sino también “simultáneamente” en otros lugares no relacionados con aquel, cuyo rastro no proporcionó más pistas.
Por ello, los expertos creen que deben examinarse muestras de sangre en otras provincias de China y en otros países, así como colonias de murciélagos en diferentes regiones asiáticas o incluso en otras partes, ha recalcado sobre lo que ha definió como un “trabajo en curso”.
Un origen fuera de Wuhan
En la rueda de prensa, Liang Wannian, el jefe del equipo de expertos chinos que investigó el SARS-CoV-2, fue un poco más allá y aseguró que pudo haber circulado en otros lugares antes que en Wuhan.
“En los dos meses previos a diciembre no hay pruebas de que estuviese en la ciudad”, afirmó Liang y destacó que la circulación temprana del coronavirus se produjo varias semanas antes de que se detectaran los primeros casos, lo que “puede explicar el fallo de su detección en otras regiones” fuera de China.
El epidemiólogo, que ejerció también de responsable del equipo designado por China para combatir la epidemia, afirmó que la investigación de las muestras de sangre y de anticuerpos de los pacientes en Wuhan en la segunda mitad de 2019 “no indican una actividad temprana del virus en la ciudad”.
Según dijo, todas las muestras de productos animales del mercado de Huanan fueron negativas en los test realizados, por lo que “no es posible determinar cómo el virus llegó hasta allí”.
Embarek dijo que el equipo había identificado a los vendedores del mercado que vendían productos animales congelados, incluidos animales salvajes de granja. “Por tanto, existe el potencial de seguir siguiendo este ejemplo y observar más a fondo la cadena de suministro y los animales que se suministraron al mercado”.