Medicina de Família
The Challenges of Antiviral Treatments
Physician Claudette Poole doesn’t take long to rattle off a list of antiviral medications she prescribes to her patients. “There really aren’t very many,” says Poole, a pediatric infectious disease physician at the University of Alabama at Birmingham.

And for people with Covid-19, there’s just one approved for use: remdesivir, which doesn’t seem to save lives, but speeds recovery in those who do get well. Clearly, more antivirals would be nice to have — so why don’t we have them? Inventing them, it turns out, is not so easy.
Viruses rely on human cell machinery to copy themselves, so antiviral designers face a challenge: how to stop the virus without damaging the inner workings of healthy cells too. While scientists have found several solutions to the problem, the antiviral pharmacopeia still lags behind the plethora of antibiotics available to treat bacterial infections.
But as researchers build up their knowledge of viral life cycles, antivirals may be poised to catch up. Scientists are also planning for future pandemics, in the hopes of having a better selection of antivirals to try the next time around.
Here’s where antivirals stand today, and how the list might be growing.
How Do Antivirals Work?
An antiviral drug can block any of the steps a virus uses to copy itself. To do its dirty work, a virus must attach to a host cell, sneak inside and trick that cell into copying viral genes and crafting viral proteins; after that, the newly made viruses must escape to infect new targets. At each step, viral genes or proteins need to interact with various host molecules, and each of these interactions offers an opportunity for antiviral drugs. The drugs often mimic those host molecules and act as decoys to interfere with the viral life cycle and reduce its spread.https://4124fa68669c2e6429bf5d79b5331880.safeframe.googlesyndication.com/safeframe/1-0-37/html/container.html
A common approach is to interfere with the copying of viral genes into DNA or RNA to form new viral genomes. Viruses frequently have their own versions of proteins, called polymerases, for this task. The polymerases add individual building blocks called nucleotides, one by one, to the new genome as it’s being built.
For example, the drug acyclovir, used to treat herpes, goes after this genome-copying step. To the virus’s polymerase, the medicine looks like just another building block — but it’s not. Once the decoy gets into the growing strand, it prevents the addition of any more nucleotides. For the virus, it’s game over.
Another drug, oseltamivir (Tamiflu) for influenza, acts at the stage of viral exit from the infected cell. The virus uses a key protein called neuraminidase to dissolve its way out, but oseltamivir sticks to the neuraminidase and stops it from working.
Since antivirals don’t eradicate viruses directly — they just stop them from spreading cell to cell or person to person — it’s up to the body’s immune system, when possible, to mop up the invaders already present. That’s why it’s important to start antiviral treatment early, while viral numbers remain low. “The faster you can take the drug, the more you can limit the virus’s ability to spread,” says virologist Mark Heise of the University of North Carolina at Chapel Hill. Tamiflu, for example, works best when taken within 48 hours of the first symptoms, helping people recover about a day faster.
Why Are There So Few Antivirals?
The number of antivirals is paltry compared with the list of bacteria-fighting antibiotics. That’s due to several factors.
For one, antibiotics were first out of the starting gate, notes Erik De Clercq, an emeritus professor of biomedicine at KU Leuven in Belgium. The first, penicillin, was discovered in 1928 and first used in a patient in 1940. In contrast, the first antiviral, idoxuridine, was developed as an anticancer agent in 1959, was reported to block viruses in 1961, and approved in 1963 to treat herpes infections of the eye. (De Clercq, a leader in early antiviral research, described his scientific journey in the Annual Review of Pharmacology and Toxicology in 2011.)
Plus, viruses are much trickier targets than bacteria, says Monica Gandhi, an infectious disease physician at the University of California, San Francisco. Bacteria are whole living cells with all the metabolic pathways they need for survival, so they offer plenty of targets for attack. They also have unique features such as cell walls that aren’t found in human cells. That means antibiotics can interfere with cell walls, or other bacteria-specific parts and processes, to kill the pathogens without harming our own cells. And because microbes evolved antibiotics to battle each other, there is a diverse array of the compounds out in nature.https://4124fa68669c2e6429bf5d79b5331880.safeframe.googlesyndication.com/safeframe/1-0-37/html/container.html
In contrast, viral pathogens live inside our own cells and depend on our proteins for most of their needs, so they offer no such easy targets. And few natural antivirals exist, so scientists need to invent them from scratch, says Kathie Seley-Radtke, a medicinal chemist at the University of Maryland, Baltimore County.
Moreover, antivirals have a limited number of possible shapes. That’s because, to block a virus’s actions, they must fit into viral proteins as decoys.
The biggest challenge, says Seley-Radtke, is to ensure that the drugs don’t hurt the human hosts as well. For example, in the case of nucleotide mimics like acyclovir, wouldn’t there be a risk that they would get into the cell’s DNA as well as the virus’s?
There are ways around that problem. In the case of acyclovir, the drug that patients swallow is an inactive form, and it is mainly activated by a viral protein. “It targets the virus very nicely, while leaving host cell DNA alone,” says Poole, who reviewed the use of acyclovir and other antivirals for newborns with herpes simplex virus in the 2018 Annual Review of Virology.
There is one unfortunate similarity between antibiotics and antivirals: In both cases, pathogens can make tiny changes to their genes and proteins that leave them unharmed by the drug. “Resistance is a huge issue in antivirals,” says Poole. Doctors used to prescribe drugs called adamantanes to people with the flu, for example — but the influenza viruses circulating among people today are unaffected by the drugs. “They are no longer effective at all,” says Poole. “There’s a desperate need to find other flu antivirals.”
The one exception to the dearth of antivirals is the flourishing pharmacopeia of meds against the human immunodeficiency virus, resulting from decades of research. Gandhi says that she can select from 30 or so medications to treat her HIV-positive patients. More are on the way — and just as well, because HIV can quickly evolve resistance to any one drug.
“HIV research set the tone,” says Heise, and it’s bringing other antivirals in its wake. “We’re seeing, again, new antivirals coming out much more quickly over the last several years.” In the last five years, for example, novel medications have turned hepatitis C from a chronic to curable condition.
“I think we’ll see more drugs for acute viral infections coming forward as well,” says Heise.
How Are Antivirals Being Used Against Covid-19?
Drugs designed against one virus often work against others, because proteins such as the polymerases used to copy virus genomes are similar across a wide range of viruses. But the current scourge requires more than the usual amount of cleverness from antiviral designers.
“Coronaviruses are pretty tricky,” Seley-Radtke says. Simple nucleotide mimics like acyclovir won’t work, because these viruses have another protein that acts as an editor, monitoring the polymerase’s work, recognizing the decoy and cutting it out.
Enter remdesivir, which had already been tested in people with Ebola (though it didn’t help them much). It’s a nucleotide mimic, but it’s a bit special. Once incorporated into a piece of the new viral genome — which in the case of coronaviruses is made of RNA — it doesn’t stop the strand’s growth right away. The polymerase keeps adding normal nucleotides. But after it’s added a few, the drug bends the RNA strand so badly that the polymerase can’t keep building. By then, though, the coronavirus editor protein no longer works; the normal nucleotides added after remdesivir seem to get in the way, says Seley-Radtke. Thus, the polymerase is stuck.
Despite that clever trick, remdesivir’s performance in people with Covid-19 has been decidedly lukewarm. On the plus side: In a trial of 1,062 people hospitalized with the virus, those who were treated with remdesivir recovered more quickly than those who received an inactive placebo. Based on this and two similar studies, the US Food and Drug Administration authorized remdesivir to treat hospitalized patients.
But what remdesivir doesn’t seem to do is save lives. In November, the World Health Organization, citing its own larger but preliminary study, recommended against remdesivir for hospitalized patients for the time being, until more research can be done. The WHO noted “no evidence that remdesivir improves survival and other outcomes.”
The results, which at first blush may seem confusing, make sense considering how antivirals work, says Poole. Because remdesivir requires multiple intravenous infusions, it’s given only to hospitalized patients. But by the time a person with Covid-19 is ill enough to be hospitalized, the virus has already run rampant across their body, so remdesivir comes too late to do much good. “The game-changer,” she says, “is going to be when we find an antiviral that you can give to people orally before they get into the hospital.”https://4124fa68669c2e6429bf5d79b5331880.safeframe.googlesyndication.com/safeframe/1-0-37/html/container.html
Remdesivir’s maker, Gilead Sciences, is working on an inhaled version. And there are other antivirals in the pipeline. For example, Seley-Radtke is optimistic about another nucleotide analog, known as AT527. Under joint development by Roche and Atea, AT527 is now midway through human trials. Like remdesivir, it has delayed action, so it avoids being edited out of a growing RNA strand. But unlike remdesivir, it’s a pill you can swallow. The companies hope it can be used by hospitalized and non-hospitalized patients alike, and perhaps even be taken by people exposed to Covid-19 to prevent infection from taking hold to begin with.
The pandemic has sent scientists scrambling to find treatments. Heise, for one, is testing a wide range of drugs — not just standard antivirals — against SARS-CoV-2 in lab dishes, as part of the Rapidly Emerging Antiviral Drug Discovery Initiative (READDI). The idea is that, because the virus depends on many processes in human cells, a variety of medications that act on human proteins might give doctors an edge by hurting the virus more than the patient. That throws the doors open to considering medications that were originally designed for cancer, psychosis, inflammatory conditions and autoimmune disease, to see if they might have a shot against Covid-19.
But the READDI collaborators — including academic centers, pharmaceutical companies and nongovernmental organizations — are aiming for more than a Covid-19 treatment. READDI hopes to identify and test potential medications for as-yet-unknown infections that may crop up in the future.
By getting early human safety testing done ahead of time, they’ll be ready to spring into action when those future outbreaks happen. As Heise says, “We don’t want to repeat what we’ve just been through.”
Amen to that.
This article is part of Reset: The Science of Crisis & Recovery, an ongoing series exploring how the world is navigating the coronavirus pandemic, its consequences and the way forward. Reset is supported by a grant from the Alfred P. Sloan Foundation.
This article originally appeared in Knowable Magazine on February 9, 2021. Knowable Magazine is an independent journalistic endeavor from Annual Reviews, a nonprofit publisher dedicated to synthesizing and integrating knowledge for the progress of science and the benefit of society. Sign up for Knowable Magazine’s newsletter.123
Knowable Magazine © 2021
Cite this: The Challenges of Antiviral Treatments – Medscape – Feb 11, 2021.
Perspectives thérapeutiques dans le syndrome cardio-rénal

Antoine FAYOL*/**, Étienne PUYMIRAT***, Marine LIVROZET*, Jean-Sébastie HULOT*,*Centre d’investigation clinique **Unité médico-chirurgicale d’insuffisance cardiaque sévère ***Unité de soins intensifs de cardiologie Hôpital européen Georges Pompidou, Paris
La prise en charge du syndrome cardio-rénal va probablement évoluer dans les années à venir grâce à l’arrivée d’un nouvel arsenal thérapeutique pour les patients insuffisants cardiaques. L’objectif de ce troisième article est de présenter ces nouvelles classes thérapeutiques qui arrivent progressivement sur le marché : les gliflozines, la finérénone, le vériciguat ou l’omecamtiv mecarbil. Ils ont très probablement une place à trouver dans la prise en charge des syndromes cardio-rénaux.
Une meilleure organisation
En 2019, l’AHA recommande la création d’unités spéciales pour la prise en charge des patients ayant un syndrome cardio-rénal via la création de structures regroupant cardiologues et néphrologues pour proposer une prise en charge commune au patient(1).
L’identification d’un diagnostic étiologique, tant cardiologique que néphrologique, semble indispensable pour la prise en charge des patients et pour l’optimisation des thérapeutiques. Le bilan d’insuffisance cardiaque et d’insuffisance rénale doit donc être fait en même temps.
La création d’une consultation spécialisée avec une surveillance régulière de la volémie du patient pourrait limiter les récidives de décompensations cardiaques.
Les caractéristiques cliniques des patients dans les essais thérapeutiques portant sur le syndrome cardio-rénal nous permettent d’envisager l’intérêt de l’évaluation de nouvelles classes thérapeutiques. Pour rappel, la majorité des patients présentant un syndrome cardio-rénal sont des hommes avec des comorbidités (hypertension, diabète dans 50 à 60 % des cas, fibrillation atriale) et une cardiopathie ischémique avec une altération de la FEVG dans 50 à 60 % des cas(2,3).
Les gliflozines
Le bénéfice cardiovasculaire des gliflozines a d’abord été confirmé chez les patients diabétiques. Le tableau 1 récapitule les principaux essais thérapeutiques.
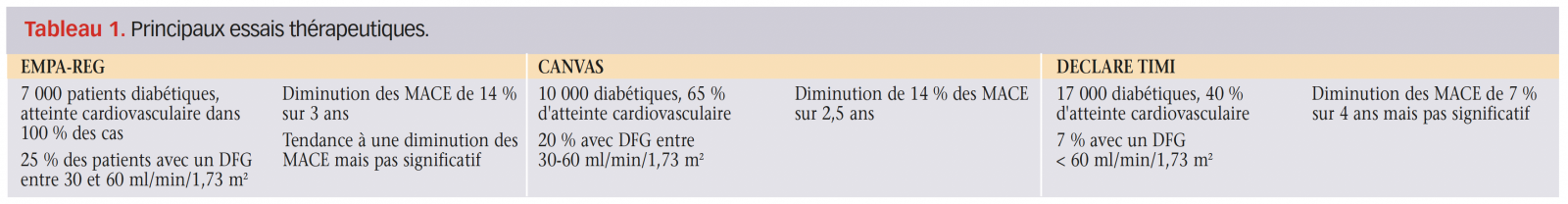
Une métaanalyse en 2019 (EMPA-REG, CANVAS, DECLARE TIMI(4-6)) s’est intéressée aux patients ayant un DFG entre 30 et 60 ml/min/1,73 m2. Pour ce groupe de patients, on observe une diminution du risque d’hospitalisation pour insuffisance cardiaque (HR 0,6), du nombre de MACE (HR 0,82) et des événements rénaux (HR 0,67).
Leurs bénéfices concernant l’insuffisance cardiaque sont également confirmés pour les patients ayant une HFrEF qu’ils soient diabétiques ou non(6,7). Dans une métaanalyse récente portant toujours sur les patients ayant une HFrEF, les patients ayant un DFG < 60 ml/min/1,73 m2 avaient une diminution significative des hospitalisations pour décompensation cardiaque et de la mortalité cardiovasculaire (HR 0,77).
Dans l’étude CREDENCE(8) (figure 1), les patients inclus étaient cette fois-ci diabétiques avec une albuminurie > 300 mg/g et un DFG entre 30 et 90 ml/ min (sous traitement conventionnel maximal). Le critère de jugement principal n’était plus cardiaque mais rénal : il s’agissait d’un critère composite d’apparition d’insuffisance rénale terminale (dialyse ou transplantation), d’un doublement de la créatinine basale ou de la mortalité cardiovasculaire et/ou rénale. L’étude a été interrompue précocement du fait d’une diminution des événements rénaux de 30 % (doublement de la créatinine ou mortalité rénale et cardiovasculaire).

Figure 1. Résultats de l’étude CREDENCE(8).
Dans l’étude DAPA-CKD (figure 2), les patients étaient diabétiques ou non avec un DFG entre 25 et 75 ml/min/1,73 m2 et une albuminurie comprise entre 0,2 g/g et 5 g/g. Le critère composite principalement rénal a lui aussi été rapidement validé (réduction de risque relatif de 37 %) avec de nouveau un arrêt précoce de l’étude(9).

Figure 2. Résultats de l’étude DAPA-CKD(9).
Enfin on peut également citer l’étude VERTIS qui s’est intéressée à l’ertugliflozine, et qui a montré qu’elle était non inférieure au placebo pour la prévention de la récidive d’événements cardiovasculaires majeurs(10).

La finérénone
La finérénone (antagoniste du récepteur aux minéralo-corticoïdes) a été évaluée chez les patients diabétiques, insuffisants rénaux dans l’étude FIDELIODKD(11) (figure 3). Au total, 5 734 patients avec un DFG compris entre 25 et 75 ml/min/1,73 m2 (avec une albuminurie > 30 mg/g) ont été inclus. Le critère de jugement principal était un critère composite associant la mortalité rénale, l’apparition d’une insuffisance rénale et une diminution de 40 % du eDFG.

Figure 3. Résultats de l’étude FIDELIO(11).
Après 2,6 ans de suivi, les patients traités par finérénone avaient une réduction significative du critère de jugement principal par rapport aux patients traités par placebo (HR 0,82 ; IC95% : 0,73-0,93 ; p = 0,001). Il y avait aussi une diminution significative du risque de survenue de MACE (AVC, infarctus du myocarde, décès d’origine cardiovasculaire) (HR 0,86 ; IC95% : 0,75- 0,99 ; p = 0,03).

Le vériciguat
Le vériciguat est un stimulateur de guanylate cyclase soluble oral, qui intervient via la voie de la guanosine monophosphate (GMP) cyclique. Il favorise la sensibilité de la guanylate cyclase soluble à l’oxyde nitrique (NO) endogène (stabilise la liaison) ce qui entraîne une vasorelaxation et une inhibition de la prolifération des muscles lisses(12).
Il a été evalué chez des patients avec une insuffisance cardiaque à FE réduite. Au total, 10 % d’entre eux avaient un DFG estimé à moins de 30 ml/min/m2 et 42 % entre 30 et 60 ml/min/m2. Le critère de jugement principal était composite associant la mortalité cardiovasculaire et la survenue d’une hospitalisation pour insuffisance cardiaque (figure 4).
Figure 4. Résultats du critère de jugement principal et dans les analyses de sous-groupe en fonction du DFG(13).
Le critère de jugement principal est survenu chez 35,5 % des patients traités et chez 38,5 % des patients ayant reçu le placebo (HR 0,90 ; IC95% : 0,82- 0,98 ; p = 0,02). Les analyses en sous-groupe n’étaient pas en faveur de l’utilisation du vériciguat chez les patients ayant un DFG < 30 ml/min/1,73 m2.

L’omecamtiv mecarbil
L’omecamtiv mecarbil(14) est un activateur direct de la myosine cardiaque, agissant directement au niveau du sarcomère, ce qui a comme effet d’augmenter le volume d’éjection systolique et de favoriser le remodelage du ventricule gauche.
Il a été évalué chez 8 256 patients avec une FEVG altérée (FEVG ≤ 35 %), ayant été hospitalisés pour un épisode de décompensation cardiaque dans l’année. Le DFG médian des patients inclus était de 58 ml/min/1,73 m2. Le critère de jugement principal était composite associant la mortalité d’origine cardiovasculaire et la survenue d’une hospitalisation pour insuffisance cardiaque.
Après 21,8 mois de suivi (figure 5), on observe une diminution significative du risque de survenue du critère composite chez les patients traités par omecamtiv mecarbil (1 523/4 120 patients [37,0 %] vs 1 607/4 112 patients [39,1 %] — HR 0,92 ; IC95% : 0,86-0,99 ; p = 0,03) ; sans diminution de la mortalité cardiovasculaire (HR 1,01 ; IC95% : 0,92- 1,11).
Figure 5. Résultats de l’étude et dans les sous-groupes d’intérêts GALACTIC-HF(14).
Les analyses en sous-groupes sont en faveur d’une efficacité plus importante du traitement chez les patients ayant une FEVG < 28 % (HR 0,84 ; IC95% : 0,77-0,92).

En pratique
▸ Une coopération entre cardiologues et néphrologues semble essentielle pour une prise en charge optimale des patients ayant un syndrome cardio-rénal. La création d’unités spéciales dédiées aux syndromes cardio-rénaux permettrait de faciliter la mise en place de thérapeutiques personnalisées.
▸ Les essais randomisés évaluant les nouvelles thérapeutiques disponibles sont nécessaires pour les patients ayant une insuffisance rénale chronique stade 4 et 5 et restent à prévoir.
Références
Cliquez sur les références et accédez aux Abstracts sur 
1. Janani R et al. Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation 2019 ; 139 : e840-e878. Rechercher l’abstract
2. Bart BA et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012 ; 367 : 2296-304. Rechercher l’abstract
3. Costanzo MR et al. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol 2007 ; 49 : 675-83. Rechercher l’abstract
4. Zinman B et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015 ; 373 : 2117-28. Rechercher l’abstract
5. Lytvyn Y et al. Sodium glucose cotransporter-2 inhibition in heart failure. Circulation 2017 ; 136 : 1643-58. Rechercher l’abstract
6. Wiviott SD et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019 ; 380 : 347-57. Rechercher l’abstract
7. McMurray JJV et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019 ; 381 : 1995-2008. Rechercher l’abstract
8. Perkovic V et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019 ; 380 : 2295-306. Rechercher l’abstract
9. Heerspink HJL et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020 ; 383 : 1436-46. Rechercher l’abstract
10. Cannon CP et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med 2020 ; 383 : 1425-35. Rechercher l’abstract
11. Bakris GL et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 2020 ; 383 : 2219-29. Rechercher l’abstract
12. Stasch JP et al. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation 2011 ; 123 : 2263-73. Rechercher l’abstract
13. Armstrong PW et al. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med 2020 ; 382 : 1883-93. Rechercher l’abstract
14. Teerlink JR et al. Cardiac myosin activation with omecamtiv mecarbil in systolic heart failure. N Engl J Med 2020 Nov 13. Rechercher l’abstract
Dieta vegetariana ou cetogênica?

Para controlar a fome, uma dieta vegetariana e com baixo teor de gordura tem vantagens em relação a uma dieta cetogênica baseada no consumo de proteína/gordura animal e com baixo teor de carboidratos, mas a dieta cetogênica ganha quando se trata de maior controle dos níveis de glicemia e insulina pós-prandiais, segundo uma nova pesquisa.
Em um estudo cruzado e cuidadosamente controlado, conduzido pelo National Institutes of Health (NIH), as pessoas consumiram menos calorias diárias quando seguiram uma dieta vegetariana e com baixo teor de gordura, mas os níveis de insulina e glicemia foram mais altos em comparação aos que fizeram uma dieta cetogênica baseada no consumo de proteína/gordura animal.
“Existe uma ideia um tanto desatualizada de que dietas com alto teor de gordura, por terem mais calorias por grama, tendem a fazer as pessoas comerem em excesso – o chamado modelo de consumo passivo excessivo”, disse ao Medscape o pesquisador sênior do estudo, Dr. Kevin Hall, Ph.D., National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) dos Estados Unidos.
O outro modelo, mais popular hoje em dia, explicou o pesquisador, é o modelo carboidrato-insulina, que preconiza que seguir uma dieta rica em carboidratos e açúcares, que causam um pico nos níveis de insulina, aumentará a fome e fará com que a pessoa coma excessivamente.
Neste estudo, Dr. Kevin Hall e colaboradores testaram essas duas hipóteses uma contra a outra.
“A resposta curta é que obtivemos resultados exatamente opostos aos do modelo de carboidrato-insulina de obesidade. Em outras palavras, em vez de as pessoas comerem mais e ganharem peso e gordura corporal, elas acabaram comendo menos com essa dieta e perdendo gordura corporal em comparação com a dieta rica em gordura”, disse o Dr. Kevin.
“Porém, o modelo de consumo passivo excessivo também falhou, porque, apesar de os participantes terem feito uma dieta muito densa em energia e rica em gordura, eles não ganharam peso nem gordura corporal. Por isso, os dois modelos sobre a razão das pessoas comerem excessivamente e ganharem peso parecem ser inadequados de acordo com o nosso estudo”, disse ele ao Medscape. “Isso sugere que as coisas são um pouco mais complicadas.”
O estudo foi publicado on-line em 21 de janeiro no periódico Nature Medicine.
Prós e contras das duas dietas
Para o estudo, os pesquisadores internaram 20 adultos saudáveis sem diagnóstico de diabetes por quatro semanas seguidas no NIH Clinical Center. A média de idade dos participantes era de 29,9 anos e o índice de massa corporal (IMC) médio foi de 27,8 kg/m2.
Os participantes foram randomizados para consumir sem restrições alimentos à base de plantas, com baixo teor de gordura (10,3% de gordura, 75,2% de carboidratos) e com baixa densidade de energia (~ 1 kcal g-1) ou para consumir alimentos com baixo teor de carboidratos, proteína/gordura animal (75,8% de gordura, 10,0% de carboidratos) e alta densidade de energia (~ 2 kcal g-1) por duas semanas. Ao final deste período, os grupos foram cruzados e passaram a seguir a outra dieta por duas semanas.
As duas dietas continham cerca de 14% de proteína e foram pareadas pelo total de calorias, embora a dieta cetogênica tivesse duas vezes mais calorias por grama de alimento do que a dieta com baixo teor de gordura. Os participantes podiam comer o que e a quantidade que quisessem das refeições recebidas.
Um participante saiu do estudo devido a ocorrência de hipoglicemia na fase da dieta cetogênica. Para o desfecho primário, os pesquisadores compararam a ingestão média diária de energia sem restrições em cada período de duas semanas de dieta.
Eles observaram que a ingestão de energia da dieta com baixo teor de gordura foi menor em aproximadamente 550 a 700 kcal d-1, em comparação com a dieta cetogênica. No entanto, apesar das grandes diferenças na ingestão de calorias, os participantes não relataram diferenças na fome, prazer nas refeições ou saciedade entre as duas dietas.
Os participantes perderam peso com ambas (cerca de 1 kg a 2 kg em média), mas apenas a dieta com baixo teor de gordura levou a uma perda de gordura corporal significativa.
“Curiosamente, nossas descobertas sugerem benefícios para ambas as dietas, pelo menos em curto prazo”, disse o Dr. Kevin em um comunicado à imprensa.
“Embora a dieta vegetariana e com baixo teor de gordura ajude a reduzir a fome, a dieta cetogênica baseada no consumo de proteína/gordura animal resultou em níveis mais baixos e estáveis de insulina e glicose. Ainda não sabemos se essas diferenças seriam mantidas em longo prazo”, disse ele.
O Dr. Kevin ressaltou que o estudo não foi desenhado para fazer recomendações de dieta para perda ponderal, e os resultados poderiam ter sido diferentes se os participantes estivessem tentando ativamente perder peso.
Na verdade, eles nem sabiam do que se tratava o estudo; apenas dissemos que queríamos que seguissem as duas dietas para vermos o que aconteceria com o corpo deles quando comessem o quanto quisessem, disse ele ao Medscape.
“É difícil definir qual dieta pode ser melhor para um indivíduo. Eu acho que você pode interpretar este estudo como existindo pontos positivos e negativos para as duas dietas”, disse o Dr. Kevin.
“Tribos” das dietas
Comentando sobre o estudo para o Medscape, o Dr. Taylor Wallace, Ph.D., professor-adjunto do Departamento de Nutrição e Estudos Alimentares na George Mason University, nos EUA, disse que é importante ressaltar que “‘dieta com baixo teor de carboidratos’ ainda precisa ser definida, e existem muitas definições”.
“Nós realmente precisamos de uma definição padronizada do que constitui ‘baixo teor de carboidratos’, para que os estudos possam ser planejados e avaliados de uma forma consistente. É problemático, porque sem uma definição padrão, os pesquisadores da ‘tribo da dieta’ (cetogênica versus vegetariana) parecem sempre encontrar a resposta a seu favor”, disse o Dr. Taylor. “Este estudo parece usar menos de 20 gramas de carboidratos por dia, o que, na minha opinião, é um teor muito baixo de carboidratos.”
Talvez a ressalva mais importante, ele acrescentou, seja que no mundo real, “a maioria das pessoas não adere a dietas muito rigorosas – nem mesmo por duas semanas”.
O estudo foi financiado pelo NIDDK Intramural Research Program, com o apoio adicional do NIH de uma subvenção do National Institute of Nursing Research. Um autor recebeu remuneração por palestrar em conferências patrocinadas por fabricantes de produtos nutricionais, atua no conselho consultivo científico da Kerry Taste and Nutrition e faz parte de um consórcio acadêmico que recebeu financiamento para pesquisa da Abbott Nutrition, Nestec e Danone. O Dr. Kevin Hall e os outros autores informaram não ter conflitos de interesses relevantes. O Dr. Taylor Wallace é diretor e CEO do Think Healthy Group; editor do Journal of Dietary Supplements; e editor adjunto do Journal of the American College of Nutrition.
Nat Med. Publicado on-line em 21 de janeiro de 2021. Abstract
Medscape Notícias Médicas © 2021 WebMD, LLC
Citar este artigo: Dieta vegetariana ou cetogênica? – Medscape – 11 de fevereiro de 2021.
La exposición prenatal a #terapia antirretroviral puede contribuir a los cambios cardiacos en los bebés

NUEVA YORK, USA. Los bebés no infectados con virus de inmunodeficiencia humana expuestos a la terapia antirretroviral antes del nacimiento pueden tener cambios cardiovasculares “relevantes”, informan médicos en España.[1]
“Se observó deterioro cardiaco subclínico junto con presión arterial más alta y grosor de la íntima media carotídea más grueso en bebés expuestos al virus de inmunodeficiencia humana a los seis meses de edad. La mitad de ellos presentaba hipertensión”, informaron la Dra. Marta López Carbonell y sus colaboradores, de la Universidad de Barcelona, en Barcelona, España.
“Nuestros hallazgos respaldan posible aumento del riesgo cardiovascular en bebés no infectados por el virus de inmunodeficiencia humana que estuvieron expuestos en el útero a terapia antirretroviral”, escribieron en Clinical Infectious Diseases.
Los investigadores dieron seguimiento a 34 bebés no infectados expuestos al virus de inmunodeficiencia humana y a 53 bebés no expuestos hasta los 6 meses de edad. Los esquemas de terapia antirretroviral durante el embarazo incluyeron dos inhibidores nucleosídicos de la transcriptasa inversa (abacavir más lamivudina: 32%; emtricitabina más tenofovir: 41%, y zidovudina más lamivudina: 21%).
A la edad de 6 meses los lactantes no infectados expuestos al virus de inmunodeficiencia humana tenían paredes miocárdicas más gruesas (grosor medio de la pared septal: 5,02 frente a 3,98 mm; p < 0,001) y disfunción sistólica relativa con disminución del desplazamiento del anillo mitral (8,57 frente a 10,34 mm; p = 0,002 ) y disminución de tricúspide S’ (9,71 frente a 11,54 cm/s; p = 0,003) junto con disfunción diastólica relativa indicada por un tiempo prolongado de relajación isovolumétrica izquierda (58,57 frente a 47,94 ms; p < 0,001).
La evaluación vascular reveló presiones arteriales sistólicas (102 frente a 80 mm Hg; p < 0,001) y diastólicas (64 frente a 55 mm Hg; p = 0,045) significativamente más altas. La mitad de los niños expuestos al virus de inmunodeficiencia humana cumplió los criterios de hipertensión, en comparación con 3,77% del grupo no expuesto (p < 0,001).
Los niños expuestos al virus de inmunodeficiencia humana también tenían grosor de la íntima media carotídea medio más grueso (0,62 frente a 0,51 µm; p = 0,015).
“Estos cambios podrían permitirnos distinguirlos como población en riesgo incluso desde la vida fetal, estableciendo seguimiento estricto y más prolongado”, indicaron los autores.https://8726cd39b10dfee9ce799a8c59060cc7.safeframe.googlesyndication.com/safeframe/1-0-37/html/container.html
“Curiosamente, nuestro estudio muestra por primera vez que la hipertensión infantil podría predecirse a partir de la vida fetal mediante la evaluación de los esquemas maternos de terapia antirretroviral que contienen zidovudina o midiendo el espesor de la pared del tabique fetal mediante ecocardiografía fetal”, añadieron.
Los investigadores agregaron que sus hallazgos también “respaldan la tendencia actual de considerar los esquemas de terapia antirretroviral que contienen zidovudina durante el embarazo como alternativa, debido a la dosificación compleja y la asociación con tasas más altas de efectos adversos leves a moderados”.
Por lo tanto, “evitar los esquemas de terapia antirretroviral que contienen zidovudina durante el embarazo, promover hábitos de estilo de vida saludable y evitar otros riesgos cardiovasculares desde la niñez, podrían potencialmente prevenir eventos cardiovasculares más adelante en la vida”.
El estudio no tuvo financiamiento comercial. Los autores han declarado no tener ningún conflicto de interés económico pertinente. La Dra. López no respondió a una solicitud de comentarios al cierre de la edición.
Traducido y adaptado por el equipo de Medscape en español.
Reuters Health Information © 2021
Citar este artículo: La exposición prenatal a terapia antirretroviral puede contribuir a los cambios cardiacos en los bebés – Medscape – 9 de feb de 2021.
Una base de datos recoge más de 600 #productos sin gluten donde se valora su composición nutricional
Según la universidad, se trata del mayor análisis comparativo respecto a su composición, tanto en ingredientes como aspecto nutricional.

El grupo de investigación Alimentación y nutrición en la promoción de la salud de la Universidad CEU San Pablo, de Madrid, ha elaborado una base de datos con 629 alimentos sin gluten, de base cereal, disponibles en el mercado español, que, hasta la fecha, “representa el mayor análisis comparativo respecto a su composición, tanto en ingredientes como nutricional, con datos tomados a partir del etiquetado”, dice la propia universidad.
Elena Alonso Aperte, vicedecana de la Facultad de Farmacia del CEU y directora de este grupo, explica a CF que desde hace tiempo tienen abierta una línea de investigación centrada en “la valoración del estado nutricional de distintos colectivos de población y en el análisis de valor nutricional de los productos”. Uno de esos colectivos es el de los celiacos, que según Alonso Aperte, “tiene ciertas dificultades a la hora de seguir una dieta sin gluten”, que es el único tratamiento que tiene esta enfermedad. Así, recuerda que ya hicieron un trabajo en niños, de los cuales valoraron su dieta, estado nutricional, determinaciones bioquímicas y grasa corporal. “Entonces vimos que no hay datos de la composición de los productos sin gluten y decidimos investigar e hicimos esta base de datos con más de 600 productos sin gluten de base cereal de los que investigamos su composición. Y su composición no es fácil de conocer”.
Según la experta, esta base de datos puede servir de base para la realización de otros estudios sobre celiaquía.

La mayoría de los 629 productos alimenticios sin gluten considerados en el trabajo, realizado durante el año 2019, se incluyeron en las categorías de galletas, tartas y dulces (36,4%), seguido de pan y productos similares (24,2%) y pasta y productos similares (14%).
En su análisis, los investigadores vieron que existe una notable falta de datos en estos alimentos acerca de su composición nutricional, principalmente de su contenido en micronutrientes, como vitaminas y minerales. Hecho que justifica la necesidad de proporcionar nuevos datos en este sentido, para completar etiquetas, tablas o bases de datos de composición de alimentos.
A juicio de Alonso Aperte, esta carencia de información “no es algo intencionado de la industria alimentaria”. La razón es de otra índole. Y es que en las bases de datos sobre composición de los alimentos elaboradas por científicos (de donde se puede extraer esta información y entre las que destacan la de Moreiras y colaboradores y la base de datos on line Bedca) no se suelen incluir los alimentos especiales, como los que no tienen gluten. “Hacer estas bases de datos es algo es muy complejo”, reconoce la vicedecana de Farmacia.
Por otra parte, recuerda que la segunda fuente de información sobre composición nutricional de los productos, es el etiquetado. La ley dice que la industria solo está obligada a declarar en el etiquetado aquellos nutrientes “más importantes y con más incidencia en la salud, como la energía, las grasas, las grasas saturadas, las proteínas, los hidratos de carbonos, azúcares y sal“. “Estos los declaran, pero el resto de componentes son voluntarios”, afirma; “además, tampoco se puede declarar todo porque sería un etiquetado muy complicado”.
Suplementación o reforzar alimentos
Para Alonso Aperte, “es prioritario proporcionar estos datos a fin de poder llevar a cabo una correcta evaluación nutricional de la población celiaca en España”. Y es que, aunque aclara que la dieta de este colectivo sigue los mismos patrones que la de colectivos sanos, los celiacos presentan mayor riesgo de deficiencia en vitamina D, calcio, magnesio, hierro y ácido fólico, de ahí la importancia de conocer qué cantidades de estos micronutrientes llevan los productos sin gluten que consumen.
La solución a estas carencias pasaría a su juicio por dos medidas: la toma de suplementos alimenticios, “siempre que haya un diagnóstico previo de esas carencias para recomendarlos y pautar la suplementación adecuada” y mejorar la composición de los alimentos, “de tal manera que se pueda mejorar la ingesta de la vitamina o mineral de interés sin necesidad de un diagnóstico previo y llegando a todos los celiacos”. “Por ejemplo -continúa- añadir ácido fólico a los alimentos es una práctica muy común en algunos países, como en Estados Unidos, donde desde 1998 es obligatorio añadir ácido fólico a los productos de base cereal, con el fin de garantizar la ingesta de ácido fólico en las embarazadas para prevenir las malformaciones congénitas del tipo defectos del tubo neural. Es una fortificación que está muy ensayada y muy segura. Por tanto, si al alimento añades una cantidad que tiene que estimarse sobre la evidencia científica de esa vitamina, que normalmente no es tanto como la suplementación, sí consigues llegar a todos los celiacos a través de los alimentos y prevenir las situaciones de riesgo”.
Calidad del perfil nutricional, a debate
Otro debate abierto es sobre la calidad del perfil nutricional de los productos sin gluten. Sobre esta cuestión el trabajo del CEU arroja que gran parte de estos alimentos mostraron contenidos muy altos de energía (33,5%), grasas (28,5%), ácidos grasos saturados (30,0%), azúcares (21,6%) y sal (28,3%). Los productos estudiados se componían principalmente de harina de arroz y/o harina de maíz, y el 90% de ellos incluían almidón de arroz añadido.
La grasa añadida más común fue el aceite de girasol (presente en un tercio de los productos), seguido de lagrasa de palma, el aceite de oliva y el cacao. Solo el 24,5% de los productos tenía la declaración nutricional sin azúcar añadido. El 56% por ciento de los productos sin gluten empleaban la sacarosa en su formulación.
No obstante, el análisis de macronutrientes reveló que el 25,4% de los productos podrían etiquetarse como fuente de fibra. Además, en comparación con el estudio realizado en 2016, empieza a apreciarse una tímida reformulación en la composición de grasas y la reducción de sal, pero un menor uso de harinas alternativas y pseudocereales, que presentan un mejor perfil nutricional.
Más oferta y variedad
Para la experta, en España la industria alimentaria ha mejorado su oferta de productos sin gluten aptos para celiacos, mejorando aspectos como la variedad y la accesibilidad, dos viejas reivindicaciones del colectivo celiaco. Según sus datos, en 2017 España fue líder en aumentar su producción de alimentos sin gluten (18,8%), en comparación con Europa occidental (13,6%) y el resto del mundo (15,4%), siendo el tercer productor mundial de este tipo de productos, después de Estados Unidos y Brasil.
Las razones de este crecimiento no se deben solo al consumo de los productos sin gluten por parte de los celiacos, condición que alcanza al 1% de la población general del mundo occidental, también es impulsado por cambios en las actitudes del consumidor hacia la salud, como la tendencia a consumir alimentos libres de.
Para la experta, hay que tener cuidado con estas modas de consumir alimentos sin gluten cuando no hay un diagnóstico de celiaquía. Y es que eso puede tener un impacto negativo en la salud y en el bolsillo del consumidor. Así, Alonso Aperte asegura que “hacer una dieta sin gluten es complicado porque hay que eliminar productos y es difícil equilibrar la dieta. Por otra parte, puede enmascarar y retrasar el diagnóstico de una celiaquía; es decir si hay una alteración intestinal y se somete a una dieta sin gluten sin que haya una recomendación médica no se va a poder diagnosticar la enfermedad celiaca. Lo mismo ocurre con los que toman productos sin latosa”. Respecto al impacto económico, recuerda que los productos sin gluten son algo más caros que sus análogos con gluten, algo que también ha criticado el colectivo de celiacos.
Otros autores
Natalia Úbeda, codirectora del proyecto, y las profesoras Violeta Fajardo, Purificación González, Lourdes Samaniego y María Achón, junto con María Martínez completan la autoría de este trabajo
Prostate Drugs Tied to Lower Risk for Parkinson’s

Certain drugs currently used to treat benign prostatic hyperplasia (BPH) may provide neuroprotection and delay or prevent the onset of Parkinson’s disease, new research suggests.
Dr Jacob Simmering
“If giving someone terazosin or similar medications truly reduces their risk of disease, these results could have significant clinical implications for neurologists,” Jacob E. Simmering, PhD, assistant professor of internal medicine at the University of Iowa in Iowa City, told Medscape Medical News.
There are few reliable neuroprotective treatments for Parkinson’s disease, he said. “We can manage some of the symptoms, but we can’t stop it from progressing. If a randomized trial finds the same result, this will provide a new option to slow progression of Parkinson’s disease,” Simmering said.
The pathogenesis of Parkinson’s disease is heterogeneous, however, and not all patients may benefit from glycolysis-enhancing drugs, the investigators note. Future research will be needed to identify potential candidates for this treatment, and clarify the effects of these drugs, they write.
The findings were published online February 1 in JAMA Neurology.
Time-Dependent Effects
The major risk factor for Parkinson’s disease is age, which is associated with impaired energy metabolism. Glycolysis is decreased among patients with Parkinson’s, yet impaired energy metabolism has not been investigated widely as a pathogenic factor in the disease, the authors write.
Studies have indicated that terazosin increases the activity of an enzyme important in glycolysis. Doxazosin and alfuzosin have a similar mechanism of action and enhance energy metabolism. Tamsulosin, a structurally unrelated drug, has the same mechanism of action as the other three drugs, but does not enhance energy metabolism.
In this report, the researchers investigated the hypothesis that patients who received therapy with terazosin, doxazosin, or alfuzosin would have a lower risk of developing Parkinson’s than patients receiving tamsulosin. To do that, they used healthcare utilization data from Denmark and the United States, including the Danish National Prescription Registry, the Danish National Patient Registry, the Danish Civil Registration System, and the Truven Health Analytics MarketScan database.
The investigators searched the records for patients who filled prescriptions for any of the four drugs of interest. They excluded any patients who developed Parkinson’s within 1 year of starting medication. Because use of these drugs is rare among women, they included only men in their analysis.
They looked at patient outcomes beginning at one year after the initiation of treatment. They also required patients to fill at least two prescriptions before the beginning of follow-up. Patients who switched from tamsulosin to any of the other drugs, or vice versa, were excluded from analysis.
The investigators used propensity score matching to ensure that patients in the tamsulosin and terazosin/doxazosin/alfuzosin groups were similar in terms of their other potential risk factors. The primary outcome was the development of Parkinson’s disease.
They identified 52,365 propensity score-matched pairs in the Danish registries and 94,883 pairs in the Truven database. The mean age was 67.9 years in the Danish registries and 63.8 years in the Truven database, and follow-up was approximately 5 years and 3 years respectively. Baseline covariates were well balanced between cohorts.
Among Danish patients, those who took terazosin, doxazosin, or alfuzosin had a lower risk of developing Parkinson’s vs those who took tamsulosin (hazard ratio [HR], 0.88). Similarly, patients in the Truven database who took terazosin, doxazosin, or alfuzosin had a lower risk of developing Parkinson’s than those who took tamsulosin (HR, 0.63).
In both cohorts, the risk for Parkinson’s among patients receiving terazosin, doxazosin, or alfuzosin, compared with those receiving tamsulosin, decreased with increasing numbers of prescriptions filled. Long-term treatment with any of the three glycolysis-enhancing drugs was associated with greater risk reduction in the Danish (HR, 0.79) and in the Truven (HR, 0.46) cohorts, vs tamsulosin.
Differences in case definitions, which may reflect how Parkinson’s disease was managed, complicate comparisons between the Danish and Truven cohorts, said Simmering. Another challenge is the source of the data.
“The Truven data set was derived from insurance claims from people with private insurance or Medicare supplemental plans,” he told Medscape Medical News. “This group is quite large but may not be representative of everyone in the United States. We would also only be able to follow people while they were on one insurance plan. If they switched coverage to a company that doesn’t contribute data, we would lose them.”
The Danish database, however, includes all residents of Denmark. Only people who left the country were lost to follow-up.
The results support the hypothesis that increasing energy in cells slows disease progression, Simmering added. “There are a few conditions, mostly REM sleep disorders, that are associated with future diagnosis of Parkinson’s disease. Right now, we don’t have anything to offer people at elevated risk of Parkinson’s disease that might prevent the disease. If a controlled trial finds that terazosin slows or prevents Parkinson’s disease, we would have something truly protective to offer these patients.”
Biomarker Needed
Dr Alberto Espay
Commenting on the results, Alberto J. Espay, MD, MSc, professor of neurology at University of Cincinnati Academic Health Center, Cincinnati, Ohio, was cautious. “These findings are of unclear applicability to any particular patient without a biomarker for a deficit of glycolysis that these drugs are presumed to affect,” Espay told Medscape Medical News. “Hence, there is no feasible or warranted change in practice as a result of this study.”
Pathogenic mechanisms are heterogeneous among patients with Parkinson’s disease, Espay added. “We will need to understand who among the large biological universe of Parkinson’s patients may have impaired energy metabolism as a pathogenic mechanism to be selected for a future clinical trial evaluating terazosin, doxazosin, or alfuzosin as a potential disease-modifying intervention.”
Parkinson’s is not one disease, but a group of disorders with unique biological abnormalities, said Espay. “We know so much about ‘Parkinson’s disease’ and next to nothing about the biology of individuals with Parkinson’s disease.”
This situation has enabled the development of symptomatic treatments, such as dopaminergic therapies, but failed to yield disease-modifying treatments, he said.
The University of Iowa contributed funds for this study. Simmering has received pilot funding from the University of Iowa Institute for Clinical and Translational Science. He had no conflicts of interest to disclose. Espay has disclosed no relevant financial relationships.
JAMA Neurol. Published online February 1, 2021. Full text
Medscape Medical News © 2021
Cite this: Prostate Drugs Tied to Lower Risk for Parkinson’s – Medscape – Feb 09, 2021.
Covid-19 durante a gestação pode aumentar risco de hipertensão e pré-eclâmpsia

Dra. Nigel Madden
“Isso não foi totalmente surpreendente, visto que a inflamação vem sendo implicada na patogênese tanto da doença hipertensiva gestacional como da infecção por SARS-CoV-2, portanto, uma pode exacerbar a outra”, a Dra. Nigel Madden, médica residente no Departamento de Ginecologia e Obstetrícia da Columbia University, nos Estados Unidos, disse ao Medscape após apresentar os resultados.
A doença hipertensiva gestacional ocorre em 10% a 15% de todas as gestações e representa a principal causa de morbidade e mortalidade materna e perinatal do mundo, explicou a Dra. Nigel aos participantes do encontro. Embora não esteja claro o que causa as doenças hipertensivas na gestação, de forma geral, “é possível que o estado inflamatório agudo da infecção por SARS-CoV-2 possa incitar ou exacerbar a doença hipertensiva gestacional”, comentou a Dra. Nigel.
Os pesquisadores conduziram uma revisão retrospectiva de prontuários de 1.715 pacientes que tiveram gestação única e que foram submetidas a exame por reação em cadeia da polimerase (PCR, sigla do inglês, Polymerase Chain Reaction), a partir de amostra nasofaríngea, no momento da admissão hospitalar na instituição na qual realizaram o trabalho de parto, entre março e junho de 2020. Os pesquisadores excluíram pacientes com história de hipertensão crônica.
No total, 10% das pacientes apresentaram resultado positivo para covid-19 (N = 167) e 90% apresentaram resultado negativo (N = 1.548). Houve várias diferenças basais entre os grupos. As pacientes com resultado positivo tenderam a ser mais jovens; média de idade de 28 anos versus 31 anos para as pacientes com resultado positivo. A proporção de pacientes acima de 35 anos de idade também foi maior no grupo negativo (P < 0,01). Houve diferenças significativas na composição racial dos grupos; metade do grupo positivo referiu “outras” ao indicar a própria raça. A maior disparidade basal entre os grupos foi em relação ao seguro de saúde: 73% das pacientes com resultado positivo para covid-19 usavam o Medicaid, enquanto apenas 36% das pacientes no grupo negativo para covid-19 usavam esse recurso. As que tinham plano de saúde particular foram mais propensas a apresentar resultado negativo (43%) do que positivo (25%) (P < 0,01).
Os pesquisadores definiram hipertensão gestacional como pressão sistólica ≥ 140 mmHg ou pressão diastólica ≥ 90 mmHg em duas aferições com no mínimo quatro horas de intervalo. O diagnóstico de pré-eclâmpsia exigiu pressão arterial elevada (mesmos critérios usados para definir hipertensão), além de proteinúria, caracterizada por razão proteína/creatinina ≥ 0,3 mg/dL ou proteína ≥ 300 mg na urina de 24 horas. A pré-eclâmpsia grave exigiu alterações laboratoriais específicas, edema pulmonar, sintomas de cefaleia, alterações visuais, dor torácica, dispneia ou dor no quadrante superior direito
Em relação à doença hipertensiva gestacional, a ocorrência da doença foi mais do que duas vezes maior entre as pacientes com covid-19 (17,9%) em comparação com as pacientes que apresentaram resultado negativo (8,4%). O grupo positivo para covid-19 teve probabilidade significativamente maior de apresentar hipertensão gestacional e pré-eclâmpsia sem características de gravidade do que o grupo negativo para covid-19. As taxas de pré-eclâmpsia com características de gravidade não foram significativamente diferentes entre os grupos.
| Negativas para covid-19 | Positivas para covid-19 | Valor de P | |
|---|---|---|---|
| Hipertensão gestacional | 3,6% | 9,0% | < 0,001 |
| Pré-eclâmpsia | 1,3% | 3,6% | 0,03 |
| Pré-eclâmpsia grave | 3,6% | 5,4% | 0,12 |
A gravidade da doença hipertensiva não diferiu entre os grupos. As limitações do estudo incluíram o fato de o desenho ter sido retrospectivo, o pequeno número de pacientes positivas para covid-19 e o estudo ter sido conduzido em uma única instituição na cidade de Nova York, nos EUA. No entanto, a população do estudo era diversificada e a pesquisa foi realizada durante o pico de infecções no epicentro da pandemia de covid-19.
“Esse foi um estudo de grande significado clínico”, disse a Dra. Kim Boggess, médica da University of North Carolina, nos EUA, enquanto moderava a sessão. “Eu diria vocês de Nova York são os mais bem posicionados para responder a algumas questões que precisam ser respondidas a respeito do efeito da infecção pelo coronavírus na gestação”.
A Dra. Kim perguntou se o estudo avaliou associações relacionadas com a gravidade da covid-19. Apenas 10 pacientes eram sintomáticas, disse a Dra. Nigel, e apenas uma apresentou pré-eclâmpsia com características de gravidade.
A Dra. Michelle Y. Owens, médica, professora e chefe de medicina materno-fetal no University of Mississippi Medical Center, nos EUA, que também moderou a sessão, disse que os achados requerem que os médicos atentem para a possibilidade de hipertensão e transtornos hipertensivos ao atenderem pacientes positivas para covid-19.
“Além disso, essas mulheres deveriam ser instruídas em relação aos transtornos hipertensivos e sintomas comuns, a fim de facilitar o diagnóstico precoce e o tratamento, quando indicado”, disse a Dra. Michelle. “Eu acredito que isso é de particular interesse para as mulheres que não são gravemente afetadas pela covid-19, na medida em que essas alterações podem ocorrer enquanto elas estiverem em quarentena ou sendo monitoradas remotamente. Isso aumenta a necessidade de avaliação remota ou monitoramento domiciliar da pressão arterial materna.”
As Dras. Nigel, Kim e Michelle informaram não ter conflitos de interesses.
Encontro anual de 2021 da Society for Maternal-Fetal Medicine (SMFM): Abstract 32. Apresentado em 28 de janeiro de 2021.
Medscape Notícias Médicas © 2021 WebMD, LLC
Citar este artigo: Covid-19 durante a gestação pode aumentar risco de hipertensão e pré-eclâmpsia – Medscape – 9 de fevereiro de 2021.
Interação entre telefones celulares e CDI
Arritmias ventriculares graves, que geralmente trazem risco de morte, são grande parte das vezes tratadas com cardioversores-desfibriladores implantáveis (CDI), que são compostos, de forma simplificada, por uma bateria, capacitores e um circuito de sensing/pacing. O sistema embutido no dispositivo responde a um campo magnético aplicado externamente e quando isso ocorre a terapia de choque fica suspensa temporariamente, ou seja, o aparelho não dará o choque se o paciente apresentar fibrilação ou taquicardia ventricular.
O novo aparelho iPhone 12, lançado recentemente, apresenta uma matriz circular de ímãs ao redor de uma bobina central, que faz com que seja compatível com acessórios MagSafe e possa ser realizado carregamento sem fio, de forma rápida, entre outras funções.

Interação entre as tecnologias
Recentemente houve relatos de possível interação do telefone com aparelhos de CDI que seria decorrente de um forte campo magnético gerado pelo Iphone e tecnologia MagSafe. Isso foi confirmado em experimento em que o telefone foi aproximado da região torácica esquerda de paciente com CDI e houve suspensão imediata das terapias do aparelho, que persistiu durante todo o teste. Esse experimento foi reproduzido diversas vezes com o telefone em diferentes posições, com o mesmo efeito. Aparelhos mais antigos não tem essa capacidade.
Também foi feito experimento randomizado de forma cega, com crossover que comparou o iPhone 12 com o iPhone XS (que não contém a tecnologia MagSafe). Os telefones eram colocados em envelopes, aproximados a menos de 2 cm do CDI por pelo menos 30 segundos e era feita avaliação do CDI. Não houve interação do iPhone XS com o CDI, porém o iPhone 12 causou suspensão da detecção de arritmias e inibição da terapia em dispositivos de diferentes marcas. Além disso, fez com houvesse reprogramação do marca-passo para modo assíncrono em um aparelho de marca específica.
Conclusão
As consequências da interação de telefones com essa nova tecnologia e aparelhos de CDI podem ser catastróficas. Como maneira de prevenir que isso ocorra, devemos reforçar ainda mais aos pacientes que usam esses dispositivos que não devem colocar os telefones próximos ao CDI, assim como qualquer outro dispositivo eletrônico que possa ter potencial de causar alterações semelhantes.
Autor(a):
Isabela Abud
Editora de cardiologia do Portal PEBMED ⦁ Graduação em Medicina pela Escola Paulista de Medicina da Universidade Federal de São Paulo (UNIFESP) ⦁ Residência em Clínica Médica pela UNIFESP ⦁ Residência em Cardiologia pelo Instituto do Coração (InCor) do Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (USP) ⦁ Atualmente atuando nas áreas de terapia intensiva, cardiologia ambulatorial, enfermaria e em ensino médico.
Referências bibliográficas:
- Greenberg JC, et al. Letter to the Editor—Lifesaving Therapy Inhibition by Phones Containing Magnets. 2021 Jan 5;S1547-5271(20)31227-3. doi: 1016/j.hrthm.2020.12.032.
- Patterson Z, et al. Letter to the Editor: New phones, old problem? Interference with cardiovascular implantable electronic devices by phones containing magnets. Heart Rhythm. 2021 Feb 2;S1547-5271(21)00105-3. doi: 1016/j.hrthm.2021.01.029.
UK COVID-19 Variant Doubling Every 10 Days in the US: Study

The SARS-CoV-2 variant first detected in the United Kingdom is rapidly becoming the dominant strain in several countries and is doubling every 10 days in the United States, according to new data.
The findings by Nicole L. Washington, PhD, associate director of research at the genomics company Helix, and colleagues were posted Sunday on the preprint server medRxiv. The paper has not been peer-reviewed in a scientific journal.
The researchers also found that the transmission rate in the United States of the variant, labeled B.1.1.7, is 30% to 40% higher than that of more common lineages.
While clinical outcomes initially were thought to be similar to those of other SARS-CoV-2 variants, early reports suggest that infection with the B.1.1.7 variant may increase death risk by about 30%.
A coauthor of the current study, Kristian Andersen, told The New York Times , “Nothing in this paper is surprising, but people need to see it.”
Andersen, a virologist at the Scripps Research Institute in La Jolla, California, added that “we should probably prepare for this being the predominant lineage in most places in the United States by March.”
The study of the B.1.1.7 variant adds support for the Centers for Disease Control and Prevention (CDC) prediction last month that it would dominate by March.https://946977d939ba33f302dec56e49d83484.safeframe.googlesyndication.com/safeframe/1-0-37/html/container.html
“Our study shows that the US is on a similar trajectory as other countries where B.1.1.7 rapidly became the dominant SARS-CoV-2 variant, requiring immediate and decisive action to minimize COVID-19 morbidity and mortality,” the researchers write.
The authors point out that the B.1.1.7 variant became the dominant SARS-CoV-2 strain in the United Kingdom within a couple of months of its detection.
“Since then, the variant has been increasingly observed across many European countries, including Portugal and Ireland, which, like the UK, observed devastating waves of COVID-19 after B.1.1.7 became dominant,” the authors write.
“Category 5” Storm
The B.1.1.7 variant has likely been spreading between US states since at least December, they write.
Medscape Medical News reported on January 15 that as of January 13, the B.1.1.7 variant was seen in 76 cases across 12 US states, according to an early release of the CDC’s Morbidity and Mortality Weekly Report (MMWR).
As of Sunday, there were 690 cases of the B.1.1.7 variant in the US in 33 states, according to the CDC.
Washington and colleagues examined more than 500,000 coronavirus test samples from cases across the United States that were tested at San Mateo, California-based Helix facilities since July.
In the study, they found inconsistent prevalence of the variant across states. By the last week in January, the researchers estimated the proportion of B.1.1.7 in the US population to be about 2.1% of all COVID-19 cases, though they found it made up about 2% of all COVID-19 cases in California and about 4.5% of cases in Florida. The authors acknowledge that their data is less robust outside of those two states.
Though that seems a relatively low frequency, “our estimates show that its growth rate is at least 35%-45% increased and doubling every week and a half,” the authors write.
“Because laboratories in the US are only sequencing a small subset of SARS-CoV-2 samples, the true sequence diversity of SARS-CoV-2 in this country is still unknown,” they note.
Michael Osterholm, PhD, MPH, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, said last week that the US is facing a “Category 5” storm with the spread of the B.1.1.7 variant as well as the variants first identified in South Africa and Brazil.
“We are going to see something like we have not seen yet in this country,” Osterholm said recently on NBC’s Meet the Press.
Lead author Nicole L. Washington and many of the coauthors are employees of Helix. Other coauthors are employees of Illumina. Three coauthors own stock in ILMN.
The work was funded by Illumina, Helix, the Innovative Genomics Institute (CYC), and the New Frontiers in Research Fund provided by the Canadian Institutes of Health Research (CYC).
Marcia Frellick is a freelance journalist based in Chicago. She has previously written for the Chicago Tribune, Science News and Nurse.com and was an editor at the Chicago Sun-Times, the Cincinnati Enquirer, and the St. Cloud (Minnesota) Times. Follow her on Twitter at @mfrellick
Medscape Medical News © 2021
Cite this: UK COVID-19 Variant Doubling Every 10 Days in the US: Study – Medscape – Feb 08, 2021.
SOLOIST-WHF démontre les bénéfices de la sotagliflozine dans l’insuffisance cardiaque aiguë du patient diabétique

Patrice DARMON, Marseille
Les inhibiteurs des co-transporteurs du sodium-glucose de type 2 (iSGLT2) ont largement démontré leurs bénéfices dans la prévention des hospitalisations pour insuffisance cardiaque chez les patients diabétiques de type 2 (DT2) à haut et très haut risque cardiovasculaire et dans le traitement de l’insuffisance cardiaque à fraction d’éjection du ventricule gauche (FEVG) diminuée en présence comme en l’absence de diabète.
La sotagliflozine est un iSGLT2 inhibant également les co-transporteurs du sodium-glucose de type 1 (iSGLT1) au niveau intestinal ; à ce jour, ce double inhibiteur SGLT1/2 n’est autorisé qu’en Europe, comme traitement adjuvant du diabète de type 1 chez les patients en surpoids (IMC ≥ 27 kg/m2). L’objectif de l’étude SOLOIST-WHF était d’évaluer les bénéfices et les risques de la sotagliflozine dans le contexte particulier de l’insuffisance cardiaque aiguë chez des patients DT2. L’essai a dû être stoppé prématurément en raison du désengagement financier du sponsor durant la pandémie de la Covid-19, mais des résultats très positifs ont été présentés au cours du dernier congrès virtuel de l’American Heart Association et publiés simultanément dans le New England of Medicine.
L’étude a inclus 1 222 patients DT2 âgés de moins de 85 ans hospitalisés pour décompensation d’une insuffisance cardiaque avec nécessité d’un recours à des diurétiques en intraveineux et élévation significative des taux de peptides natriurétiques. Les critères d’exclusion étaient les suivants : insuffisance cardiaque terminale ; syndrome coronarien aigu, revascularisation coronarienne ou accident vasculaire cérébral récents ; DFGe < 30 ml/min/1,73 m2 ; instabilité clinique (définie par le besoin de recourir à une oxygénothérapie ou à un traitement inotrope ou vasodilatateur en intraveineux — à l’exception des dérivés nitrés — ou de poursuivre les diurétiques en intraveineux ou par une pression artérielle systolique < 100 mmHg). Les sujets ont été randomisés pour recevoir de la sotagliflozine (200 mg/j, pouvant être augmentée à 400 mg/j en l’absence d’effets secondaires) ou un placebo, soit avant la sortie (48,8 % des cas), soit dans les trois jours suivant la sortie (51,2 %). Les caractéristiques des patients à l’inclusion étaient les suivantes : âge médian 70 ans ; 33,7 % de femmes ; IMC 30,8 kg/m2 ; FEVG 35 % (< 50 % dans 79,1 % des cas) ; NT-proBNP 1799,7 pg/ml ; DFGe 49,7 ml/min/1,73 m2 ; HbA1c 7,1 %. Le critère primaire de jugement, modifié à l’arrêt des inclusions pour augmenter la puissance de l’étude, était un critère composite des décès d’origine cardiovasculaire et du nombre total d’hospitalisations et de visites en urgence pour insuffisance cardiaque. Au terme d’un suivi médian de seulement 9 mois, ce critère était réduit de façon significative sous sotagliflozine (51,0 vs 76,3 événements pour 100 patients-années ; HR 0,67 [IC95% 0,52-0,85], p < 0,001). Ce bénéfice était retrouvé à l’identique dans tous les sous-groupes étudiés (hommes ou femmes, âge < ou ≥ 65 ans, origine géographique des centres, traitement débuté avant ou après la sortie, FEVG < ou ≥ 50 %, DFGe < ou ≥ 60 m/min/1,73 m2). Le bénéfice de la sotagliflozine est également retrouvé sur le critère de jugement initialement prévu (décès cardiovasculaire ou première hospitalisation pour insuffisance cardiaque) avec un HR à 0,71 (IC95% 0,56-0,89). À l’inverse, la réduction observée du risque de décès d’origine cardiovasculaire et de décès de toutes origines n’était pas significative (HR 0,84 [IC95% 0,58-1,22] et HR 0,82 [IC95% 0,59-1,14], respectivement).
Les données sur la sécurité de la sotagliflozine, et plus particulièrement sur les événements liés à la déplétion volémique, étaient très attendus dans ce contexte d’insuffisance cardiaque aiguë, et elles sont rassurantes : pas de différence significative entre sotagliflozine et placebo pour les épisodes d’hypotension (6,0 % vs 4,6 %) et d’insuffisance rénale aiguë (4,1 % vs 4,4 %). Les seuls effets indésirables retrouvés plus souvent sous sotagliflozine étaient les diarrhées (6,1 % vs 3,4 %), les hypoglycémies sévères (1,5 % vs 0,3 %) et les infections génitales (0,8 vs 0,2 %) ; les épisodes d’acidocétose étaient rares et moins fréquents sous sotagliflozine que sous placebo (0,3 % vs 0,7 %).
Au final, en dépit de son interruption prématurée, cet essai démontre que la sotagliflozine a un rapport bénéfices-risques hautement favorable en cas d’insuffisance cardiaque aiguë chez le patient diabétique de type 2, et ce, quelle que soit la FEVG. Il s’agit là d’une nouvelle preuve des bénéfices de l’inhibition de SGLT2 en cas d’insuffisance cardiaque, ici dans la situation très particulière de la période post-décompensation. Il reste à déterminer si l’inhibition de SGLT1 induite par la sotagliflozine apporte un bénéfice spécifique chez ces patients.
Publié par Diabétologie Pratique
Références
Cliquez sur les références et accédez aux Abstracts sur 
Bhatt DL et al., for the SOLOIST-WHF Investigators. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 2020 Nov 16. Rechercher l’abstract
- ← Anterior
- 1
- 2
- 3
- …
- 99
- Próximo →